545953
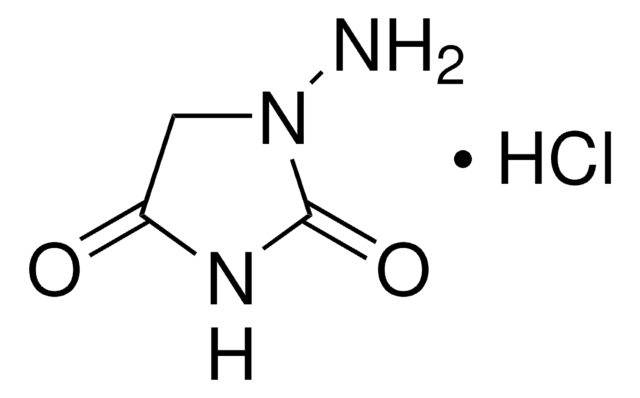
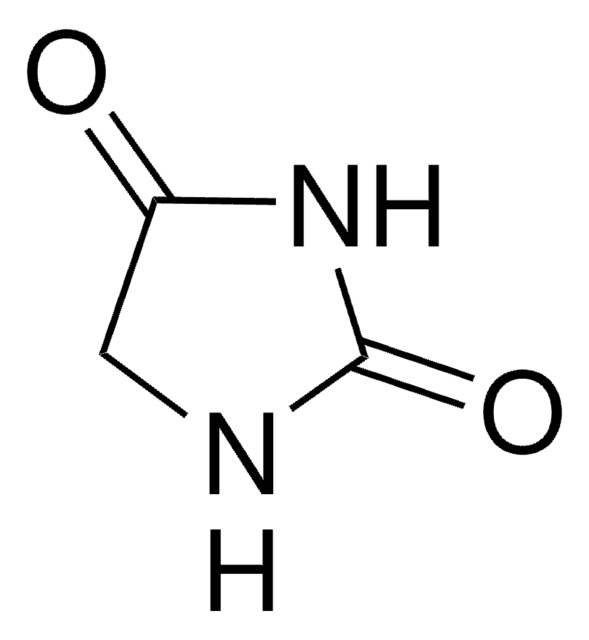
1-Aminohydantoin hydrochloride
98%
Synonym(s):
1-Amino-2,4-imidazolidinedione hydrochloride
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C3H5N3O2 · HCl
CAS Number:
Molecular Weight:
151.55
Beilstein:
3699376
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
mp
201-205 °C (lit.)
SMILES string
Cl[H].NN1CC(=O)NC1=O
InChI
1S/C3H5N3O2.ClH/c4-6-1-2(7)5-3(6)8;/h1,4H2,(H,5,7,8);1H
InChI key
WEOHANUVLKERQI-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
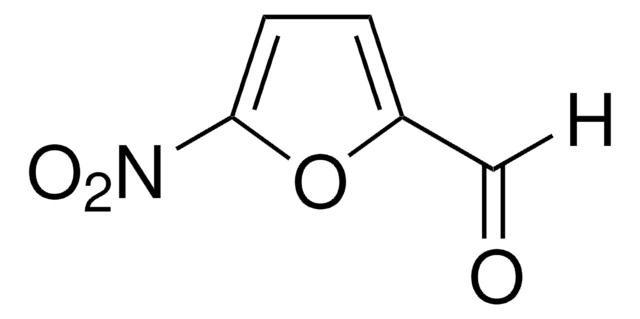
1-Aminohydantoin hydrochloride may be used as one of the reactants in the synthesis of (E)-1-(2-hydroxybenzylideneamino)imidazolidine-2,4-dione, dantrolene and dantrolene sodium.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Crystal structure of (E)-1-(2-hydroxybenzylideneamino) imidazolidine-2, 4-dione, C10H9N3O3.
Hu, Lei, et al.
Zeitschrift fur Kristallographie, 230(2), 111-112 (2015)
Johann Leban et al.
Bioorganic & medicinal chemistry, 16(8), 4579-4588 (2008-03-04)
Peptide-semicarbazones derived from Z-Trp-Trp-Phe-aldehyde inhibit the chymotryptic activity of the human proteasome at nanomolar concentrations, but are less active in a NFkappaB reporter gene assay. Cyclic semicarbazones, in contrast, combine a strong inhibitory effect on the enzyme with an inhibition
William Andrew Publishing et al.
Pharmaceutical Manufacturing Encyclopedia, 1-4, 1193-1193 (2013)
R L White et al.
Journal of medicinal chemistry, 30(2), 263-266 (1987-02-01)
A series of 1-[[[5-(substituted phenyl)-2-oxazolyl]methylene]amino]- 2,4-imidazolidinediones (6a-t) was synthesized, and the compounds were evaluated for direct skeletal muscle inhibition in the pithed rat gastrocnemius muscle preparation. The correctness of structural assignment of the new series was verified by alternate, unequivocal
Synthesis of 1-Aminohydantoin Hydrochloride-(2-~(13) C,~(15) N_3) as Double Labeling Compound
Xu JF, et al.
Chemical World, 10, 015-015 (2012)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service