All Photos(1)
About This Item
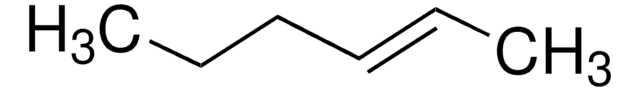
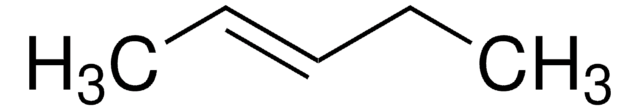
Linear Formula:
C2H5CH=CHC2H5
CAS Number:
Molecular Weight:
84.16
Beilstein:
1718859
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥99%
refractive index
n20/D 1.394 (lit.)
bp
67 °C (lit.)
density
0.677 g/mL at 25 °C (lit.)
SMILES string
CC\C=C\CC
InChI
1S/C6H12/c1-3-5-6-4-2/h5-6H,3-4H2,1-2H3/b6-5+
InChI key
ZQDPJFUHLCOCRG-AATRIKPKSA-N
Looking for similar products? Visit Product Comparison Guide
accessory
Product No.
Description
Pricing
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Asp. Tox. 1 - Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
-13.0 °F - closed cup
Flash Point(C)
-25 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Study on the separation of 1-hexene and trans-3-hexene using ionic liquids.
Jiqin Z, et al.
Fluid Phase Equilibria, 247(1), 102-106 (2006)
Electronic effects of bis (2-aryl-4, 5, 6, 7-tetrahydroindenyl) titanocene dichlorides on the catalytic epoxidation of trans-3-hexene.
Halterman RL and Ramsey TM.
Journal of Organometallic Chemistry, 465(1), 175-179 (1994)
Synthesis and ene reactions of di-(-)-menthyl diazenedicarboxylate.
Brimble MA, et al.
Tetrahedron Asymmetry, 7(7), 2007-2016 (1996)
Oligomers from the ozonolyses of cis-and trans-3-hexene and cis-and trans-2, 5-dimethyl-3-hexene
Murray RW and Su JS.
The Journal of Organic Chemistry, 48(6), 817-822 (1983)
Alla Zelenyuk et al.
Faraday discussions, 200, 143-164 (2017-06-06)
When secondary organic aerosol (SOA) particles are formed by ozonolysis in the presence of gas-phase polycyclic aromatic hydrocarbons (PAHs), their formation and properties are significantly different from SOA particles formed without PAHs. For all SOA precursors and all PAHs, discussed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service