SML1875
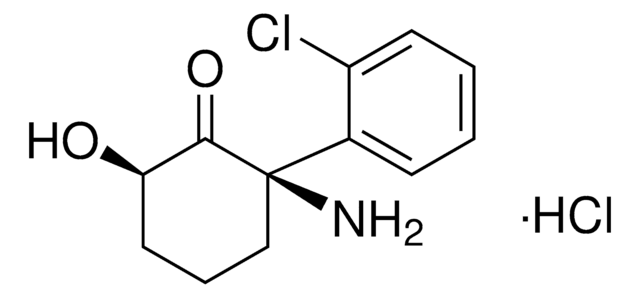
(2S,6S)-Hydroxynorketamine hydrochloride
≥98% (HPLC)
Synonym(s):
(2S,6S)-2-Amino-2-(2-chlorophenyl)-6-hydroxy-cyclohexanone hydrochloride, (2S,6S)-HNK hydrochloride
About This Item
Recommended Products
Quality Level
Assay
≥98% (HPLC)
form
powder
optical activity
[α]/D +95 to +115°, c = 1 in H2O
drug control
regulated under CDSA - not available from Sigma-Aldrich Canada
storage condition
desiccated
color
white to beige
solubility
H2O: 25 mg/mL, clear
storage temp.
2-8°C
SMILES string
[H]Cl.ClC1=CC=CC=C1[C@](CCC[C@@H]2O)(N)C2=O
Biochem/physiol Actions
It has been found that the NMDAR antagonist (R,S)-ketamine must be metabolized to (2S,6S;2R,6R)-hydroxynorketamine (HNK) to have antidepressant effects. The (2R,6R)-HNK enantiomer appears to be the enantiomer most responsible for antidepressant effects, while (2S,6S)-hydroxynorketamine was associated with increased locomotor activity and motor incoordination. (2S,6S)-HNK, was also found to increase the function of the mammalian target of rapamycin (mTOR) 2-fold at 0.05 nM. (2S,6S)-HNK and (2R,6R)-HNK both inhibited intracellular concentrations of D-serine, an endogenous NMDA receptor co-agonist, with IC50 values of 0.18 nM and 0.68 nM, respectively. Neither bind with high affinity to NMDA receptors, with Ki values of 21.19 μM and > 100 μM for (2S,6S)-HNK and (2R,6R)-HNK, respectively.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service