60372
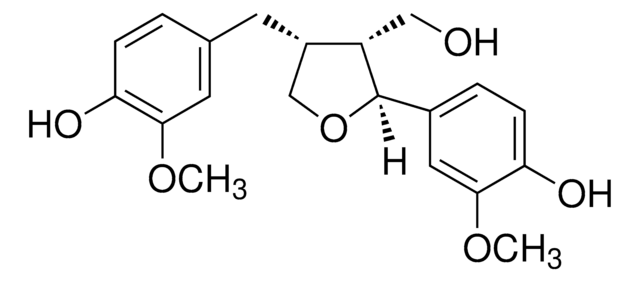
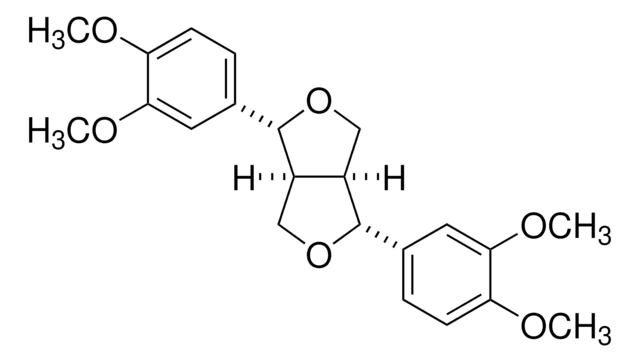
Secoisolariciresinol
≥95.0% (HPLC)
Synonym(s):
(2R,3R)-2,3-Bis(4-hydroxy-3-methoxybenzyl)-1,4-butanediol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C20H26O6
Molecular Weight:
362.42
Beilstein:
6611290
EC Number:
MDL number:
UNSPSC Code:
41116105
PubChem Substance ID:
NACRES:
NA.21
Recommended Products
Assay
≥95.0% (HPLC)
form
solid
SMILES string
OC[C@@H]([C@H](CO)CC1=CC=C(O)C(OC)=C1)CC2=CC(OC)=C(O)C=C2
InChI
1S/C20H26O6/c1-25-19-9-13(3-5-17(19)23)7-15(11-21)16(12-22)8-14-4-6-18(24)20(10-14)26-2/h3-6,9-10,15-16,21-24H,7-8,11-12H2,1-2H3/t15-,16-/m0/s1
InChI key
PUETUDUXMCLALY-HOTGVXAUSA-N
Application
Secoisolariciresinol is a metabolite of secoisolariciresinol diglucoside (SDG), an antioxidant present in flaxseed.
Packaging
Bottomless glass bottle. Contents are inside inserted fused cone.
Physical form
mixture of enantiomers of the (R*,R*)-diastereoisomer
Other Notes
Component of functional food. Flaxseed lignan with antioxidant activity
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Hisashi Nishiwaki et al.
Bioscience, biotechnology, and biochemistry, 75(9), 1735-1739 (2011-09-08)
The larvicidal activity against Culex pipiens of all stereoisomers of dihydroguaiaretic acid (DGA) and secoisolariciresinol was measured, and these DGAs were found to be potent. Sixteen (-)-DGA derivatives were then newly synthesized to analyze their structure-activity relationship. Two derivatives monohydroxylated
Carol J Fabian et al.
Cancer prevention research (Philadelphia, Pa.), 3(10), 1342-1350 (2010-08-21)
Preclinical and correlative studies suggest reduced breast cancer with higher lignan intake or blood levels. We conducted a pilot study of modulation of risk biomarkers for breast cancer in premenopausal women after administration of the plant lignan secoisolariciresinol given as
M R O'Neil et al.
Domestic animal endocrinology, 37(3), 148-158 (2009-06-30)
To evaluate the estrogenic potential of secoisolariciresinol diglycoside (SDG) found in linseed meal (LSM) on visceral organ mass, IGF-I, and thyroid hormone (T(3) and T(4)) concentrations, 48 multiparous, ovariectomized ewes (54.6 +/- 1.1 kg) were used in a 3 x
K Struijs et al.
Journal of applied microbiology, 107(1), 308-317 (2009-03-24)
It has been investigated whether secoisolariciresinol (SECO) and anhydrosecoisolariciresinol (AHS), an acid degradation product of SECO, could be fermented in a similar way, and to a similar extent, by members of the intestinal microbiota. AHS and SECO were demethylated by
Sandra M Sacco et al.
Journal of medicinal food, 14(10), 1208-1214 (2011-06-15)
Flaxseed, rich in the phytoestrogen lignan secoisolariciresinol diglycoside (SDG), provides protection against bone loss at the lumbar vertebrae primarily when combined with low-dose estrogen therapy in the ovariectomized rat model of postmenopausal osteoporosis. Whether SDG metabolites are accessible to skeletal
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service