All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C4HCl2FN2
CAS Number:
Molecular Weight:
166.97
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
mp
37-41 °C (lit.)
SMILES string
Fc1cnc(Cl)nc1Cl
InChI
1S/C4HCl2FN2/c5-3-2(7)1-8-4(6)9-3/h1H
InChI key
WHPFEQUEHBULBW-UHFFFAOYSA-N
Application
2,4-Dichloro-5-fluoropyrimidine can be used as a starting material to synthesize:
- 5-fluoropyrimidine-2-carboxamides and 5-fluoropyrimidine-4-carboxamides as potential kinase inhibitors.
- A series of 2,4-diamino-5-fluoropyrimidine derivatives as potential protein kinase Cθ inhibitors.
- 2,4-Bisanilinopyrimidine derivatives as potential aurora kinases inhibitors.
- 5-fluoro-N,N-bis(4-methoxyphenyl)-2,4-pyrimidinediamine by reacting with p-methoxy aniline in the presence of DIPEA.
- 2-chloro-5-fluoro-4-(4-fluorophenyl)pyrimidine by Suzuki coupling reaction in the presence of (4-fluorophenyl)boronic acid triphenylphosphine, and palladium(II) acetate catalyst.
- 5-fluoro-2-(piperidin-4-yloxy)pyrimidin-4-amine, a scaffold, which is used in the preparation of potent deoxycytidine kinase inhibitors.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Skin Corr. 1B - Skin Sens. 1A
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
222.8 °F - closed cup
Flash Point(C)
106 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Design, synthesis, and biological evaluation of a series of novel AXL kinase inhibitors
Mollard A, et al.
ACS Medicinal Chemistry Letters, 2(12), 907-912 (2011)
Ignacio Aliagas-Martin et al.
Journal of medicinal chemistry, 52(10), 3300-3307 (2009-05-01)
The two major Aurora kinases carry out critical functions at distinct mitotic stages. Selective inhibitors of these kinases, as well as pan-Aurora inhibitors, show antitumor efficacy and are now under clinical investigation. However, the ATP-binding sites of Aurora A and
Optimization of 2, 4-diamino-5-fluoropyrimidine derivatives as protein kinase C theta inhibitors with mitigated time-dependent drug-drug interactions and P-gp liability
Kunikawa S, et al.
Bioorganic & Medicinal Chemistry, 23(13), 3269-3277 (2015)
Facile and regioselective synthesis of novel 2, 4-disubstituted-5-fluoropyrimidines as potential kinase inhibitors
Wada H, et al.
Tetrahedron Letters, 53(14), 1720-1724 (2012)
Practical synthesis of 5-fluoro-2-(piperidin-4-yloxy) pyrimidin-4-amine, a key intermediate in the preparation of potent deoxycytidine kinase inhibitors
Zhang H, et al.
Organic Process Research & Development, 13(4), 807-811 (2009)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service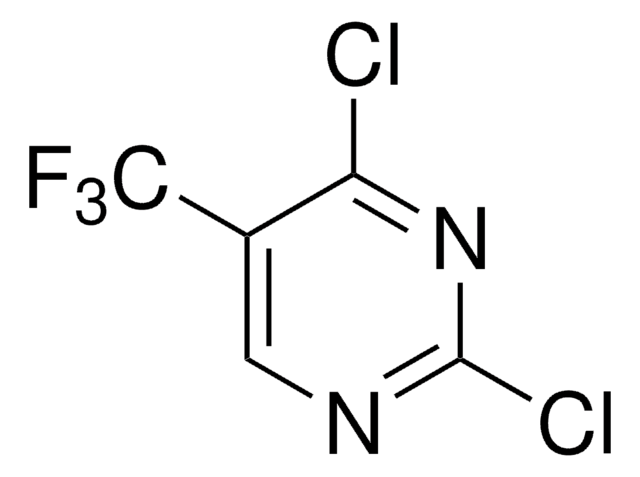
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II), complex with dichloromethane](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)