860455P
Avanti
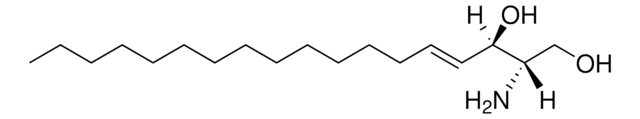
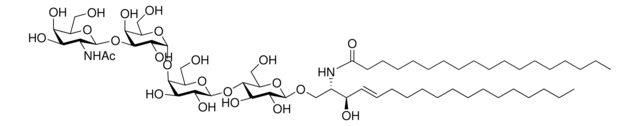
N-C12-deoxysphingosine
N-lauroyl-1-deoxysphingosine (m18:1/12:0), powder
Synonym(s):
N-dodecanoyl-1-deoxysphing-4-enine (m18:1/12:0); N-C12-1-deoxyCer; 110992
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C30H59NO2
CAS Number:
Molecular Weight:
465.79
UNSPSC Code:
12352211
NACRES:
NA.25
Recommended Products
Assay
>99% (TLC)
form
powder
packaging
pkg of 1 × 1 mg (860455P-1mg)
pkg of 1 × 5 mg (860455P-5mg)
manufacturer/tradename
Avanti Research™ - A Croda Brand 860455P
lipid type
sphingolipids
bioactive lipids
shipped in
dry ice
storage temp.
−20°C
General description
N-C12-deoxysphingosine, also known as 1-deoxydihydroceramide (1-deoxyDHCer) is, the N-acylated form of 1-deoxysphinganine, a potent inhibitor of sphingolipid metabolism.
Application
N-C12-deoxysphingosine has been used as a standard in the quantitation of atypical sphingoid bases in biological samples by reverse-phase liquid chromatography coupled to electrospray ionization tandem mass spectrometry (LC-MS/MS).
Packaging
5 mL Amber Glass Screw Cap Vial (860455P-1mg)
5 mL Amber Glass Screw Cap Vial (860455P-5mg)
Legal Information
Avanti Research is a trademark of Avanti Polar Lipids, LLC
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Nicholas C Zitomer et al.
The Journal of biological chemistry, 284(8), 4786-4795 (2008-12-20)
Fumonisin B(1) (FB(1)) is a mycotoxin that inhibits ceramide synthases (CerS) and causes kidney and liver toxicity and other disease. Inhibition of CerS by FB(1) increases sphinganine (Sa), Sa 1-phosphate, and a previously unidentified metabolite. Analysis of the latter by
Junliang Wan et al.
Journal of agricultural and food chemistry, 67(46), 12953-12961 (2019-10-23)
Most common sphingolipids are comprised of "typical" sphingoid bases (sphinganine, sphingosine, and structurally related compounds) and are produced via the condensation of l-serine with a fatty acyl-CoA by serine palmitoyltransferase. Some organisms, including mammals, also produce "atypical" sphingoid bases that
Terina N Martinez et al.
Molecular neurodegeneration, 7, 45-45 (2012-09-15)
Dopaminergic (DA) neurons in the ventral midbrain selectively degenerate in Parkinson's disease (PD) in part because their oxidative environment in the substantia nigra (SN) may render them vulnerable to neuroinflammatory stimuli. Chronic inhibition of soluble Tumor Necrosis Factor (TNF) with
Sarah T Pruett et al.
Journal of lipid research, 49(8), 1621-1639 (2008-05-24)
"Sphingosin" was first described by J. L. W. Thudichum in 1884 and structurally characterized as 2S,3R,4E-2-aminooctadec-4-ene-1,3-diol in 1947 by Herb Carter, who also proposed the designation of "lipides derived from sphingosine as sphingolipides." This category of amino alcohols is now
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service