CDS010244
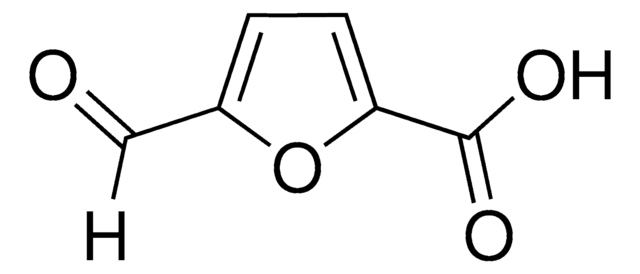
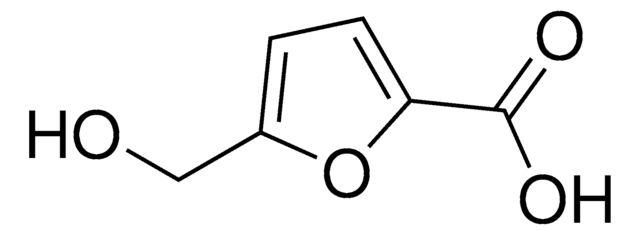
5-hydroxymethyl-2-furancarboxylic acid
AldrichCPR
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H6O4
CAS Number:
Molecular Weight:
142.11
MDL number:
UNSPSC Code:
12352106
PubChem Substance ID:
Recommended Products
SMILES string
OCc1ccc(o1)C(O)=O
InChI
1S/C6H6O4/c7-3-4-1-2-5(10-4)6(8)9/h1-2,7H,3H2,(H,8,9)
InChI key
PCSKKIUURRTAEM-UHFFFAOYSA-N
Other Notes
Please note that Sigma-Aldrich provides this product to early discovery researchers as part of a collection of unique chemicals. Sigma-Aldrich does not collect analytical data for this product. Buyer assumes responsibility to confirm product identity and/or purity. All sales are final.
NOTWITHSTANDING ANY CONTRARY PROVISION CONTAINED IN SIGMA-ALDRICH′S STANDARD TERMS AND CONDITIONS OF SALE OR AN AGREEMENT BETWEEN SIGMA-ALDRICH AND BUYER, SIGMA-ALDRICH SELLS THIS PRODUCT "AS-IS" AND MAKES NO REPRESENTATION OR WARRANTY WHATSOEVER WITH RESPECT TO THIS PRODUCT, INCLUDING ANY (A) WARRANTY OF MERCHANTABILITY; (B) WARRANTY OF FITNESS FOR A PARTICULAR PURPOSE; OR (C) WARRANTY AGAINST INFRINGEMENT OF INTELLECTUAL PROPERTY RIGHTS OF A THIRD PARTY; WHETHER ARISING BY LAW, COURSE OF DEALING, COURSE OF PERFORMANCE, USAGE OF TRADE OR OTHERWISE.
NOTWITHSTANDING ANY CONTRARY PROVISION CONTAINED IN SIGMA-ALDRICH′S STANDARD TERMS AND CONDITIONS OF SALE OR AN AGREEMENT BETWEEN SIGMA-ALDRICH AND BUYER, SIGMA-ALDRICH SELLS THIS PRODUCT "AS-IS" AND MAKES NO REPRESENTATION OR WARRANTY WHATSOEVER WITH RESPECT TO THIS PRODUCT, INCLUDING ANY (A) WARRANTY OF MERCHANTABILITY; (B) WARRANTY OF FITNESS FOR A PARTICULAR PURPOSE; OR (C) WARRANTY AGAINST INFRINGEMENT OF INTELLECTUAL PROPERTY RIGHTS OF A THIRD PARTY; WHETHER ARISING BY LAW, COURSE OF DEALING, COURSE OF PERFORMANCE, USAGE OF TRADE OR OTHERWISE.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Rajendran Omana Rajesh et al.
Bioresource technology, 284, 155-160 (2019-04-01)
The aim of the present study was to develop an eco-friendly biological process for the production of 2,5-furan dicarboxylic acid (FDCA) from 5-hydroxy methylfurfuraldehyde (HMF) using microorganisms. Microorganisms were isolated from the soil samples and evaluated for its biotransformation efficiency.
Hiram F Ramírez-Cahero et al.
Food chemistry, 245, 1131-1140 (2017-12-31)
The radiolytic decomposition of glucose, fructose, sucrose, ascorbic acid (H
Zi-Wei Wang et al.
Bioresource technology, 303, 122930-122930 (2020-02-11)
The main aim of this work was to firstly develop a selective oxidation approach for biologically converting 5-hydroxymethylfurfural and furfural into the corresponding furan-based carboxylic acids with recombinant Escherichia coli HMFOMUT. Whole-cells of this recombinant strain harbored good biocatalytic activity
Kasanneni Tirumala Venkateswara Rao et al.
ChemSusChem, 11(18), 3323-3334 (2018-07-15)
A highly active and inexpensive Co-Mn mixed-oxide catalyst was prepared and used for selective oxidation of 5-hydroxymethylfurfural (HMF) into 2, 5-furandicarboxylic acid (FDCA). Co-Mn mixed-oxide catalysts with different Co/Mn molar ratios were prepared through a simple solid-state grinding method-a low-cost
Maria Ventura et al.
ChemSusChem, 11(8), 1305-1315 (2018-03-08)
Mixed oxides based on MgO⋅CeO2 were used as efficient catalysts in the aerobic oxidation of 5-hydroxymethylfurfural (5-HMF) to afford, with very high selectivity, either 2,5-diformylfuran (DFF, 99 %) or 2-formyl-5-furancarboxylic acid (FFCA, 90 %), depending on the reaction conditions. 5-Hydroxymethyl-2-furancarboxylic acid (HMFCA
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service