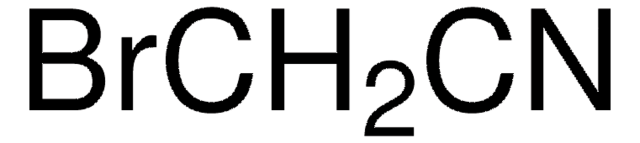All Photos(1)
About This Item
Linear Formula:
Cl3CCONH2
CAS Number:
Molecular Weight:
162.40
Beilstein:
1754028
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
Recommended Products
Assay
99%
form
solid
bp
238-240 °C (lit.)
mp
139-141 °C (lit.)
SMILES string
NC(=O)C(Cl)(Cl)Cl
InChI
1S/C2H2Cl3NO/c3-2(4,5)1(6)7/h(H2,6,7)
InChI key
UPQQXPKAYZYUKO-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Trichloroacetamide is the major degradation product of trichloroacetonitrile.
Application
Trichloroacetamide was used in microarray-based transcriptomics and one-dimensional proton nuclear magnetic resonance based metabonomics to investigate the health effects of nitrogenous disinfection byproducts of trichloroacetamide in mice.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Peter J Meloncelli et al.
Carbohydrate research, 346(12), 1406-1426 (2011-05-03)
The ABO histo-blood group antigens are best known for their important roles in solid organ and bone marrow transplantation as well as transfusion medicine. Here we report the synthesis of the ABO type III and IV antigens with a 7-octen-1-yl
Jeffrey S Arnold et al.
Chemical communications (Cambridge, England), 48(94), 11531-11533 (2012-10-24)
We report the chiral diene ligated rhodium-catalyzed dynamic kinetic asymmetric transformation (DYKAT) of racemic secondary allylic trichloroacetimidates with a variety of N-methyl anilines, providing allylic N-methyl arylamines in high yields, regioselectivity, and enantiomeric excess. The rhodium-catalyzed DYKAT method addresses limitations
Jeffrey S Arnold et al.
Organic letters, 12(20), 4580-4583 (2010-09-17)
The use of unactivated aromatic amines in the rhodium-catalyzed regioselective amination of secondary allylic trichloroacetimidates is explored. The desired N-arylamines are obtained in high yields and regioselectivity, favoring the branched amination products. The presence of the trichloroacetimidate leaving group was
Yan Zhang et al.
Environmental science & technology, 47(6), 2918-2924 (2013-02-15)
Microarray-based transcriptomics and one-dimensional proton nuclear magnetic resonance ((1)H NMR) based metabonomics approaches were employed to investigate the health effects of nitrogenous disinfection byproducts (N-DBPs) of trichloroacetamide (TCAcAm) on mice. Mice were exposed to TCAcAm at concentrations of 50, 500
Nihan Celebi-Olçüm et al.
The Journal of organic chemistry, 74(18), 6944-6952 (2009-08-20)
Density functional theory calculations were used to investigate the [3,3]- and [1,3]-shifts of O-allylic trichloroacetimidates in the presence of cinchona alkaloids. Thermal [1,3]- and [3,3]-rearrangements proceed through concerted pseudopericyclic transition states to give the corresponding rearranged products. [1,3]-Rearrangement is catalyzed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![1,8-Diazabicyclo[5.4.0]undec-7-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)