Lipshutz Surfactants Portfolio
Introduction
Micellar catalysis has emerged as a sustainable mode of chemical synthesis, providing an effective alternative to environmentally egregious and waste-generating organic solvents, replacing them with an aqueous medium containing very limited amounts of a benign-by-design nonionic surfactant. These amphiphiles self-assemble into nanomicelles that host substrates and reagents, and/or catalysts, within their lipophilic cores, enabling their solubilization within a bulk aqueous medium while simultaneously protecting them from interactions with the surrounding water. Thus, reactions involving otherwise water-sensitive reagents (e.g., the in situ formation of organozinc reagents) become possible. Furthermore, the characteristically high concentrations within nanomicelles (typically ca. 2 M) often leads to higher reaction rates than when performed in organic solvents.
Over the last 15 years, the laboratories of Prof. Bruce Lipshutz have engineered a series of “designer” surfactants (so-named as they have been developed to maximize efficiency for reactions performed in water). These are listed below, and range from general purpose amphiphiles to those earmarked for specific needs.
To learn more about micellar catalysis download our brochure.
PTS
This 1st generation surfactant (Figure 1) continues to play an important role in micellar catalysis, as it serves as a testing ground that enables many of the earlier processes, first run in water, to be evaluated.
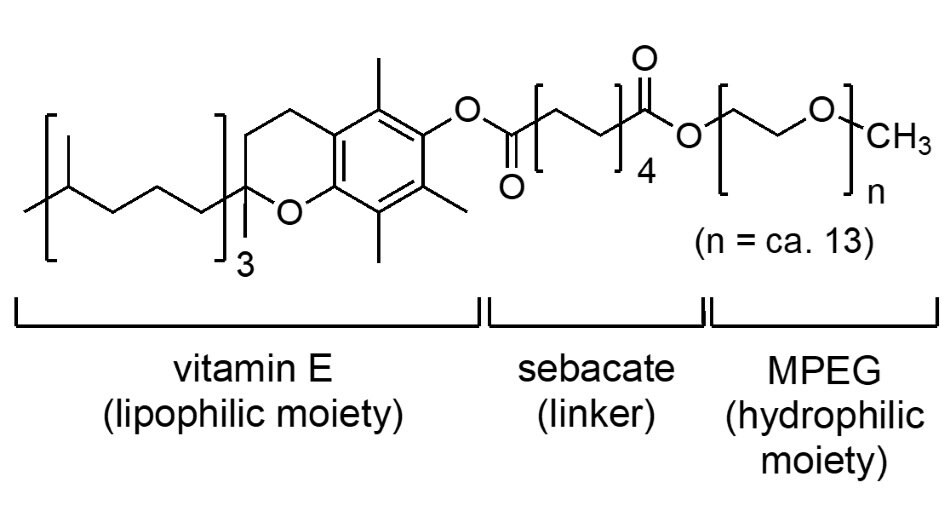
Figure 1.The structure of the surfactant PTS also known as Polyoxyethanyl-α-tocopheryl sebacate.
TPGS-750-M
This surfactant (Figure 2), as with PTS, is derived from vitamin E, but contains MPEG-750 attached via an inexpensive succinate linker. Introduced in 2011, it has long been the workhorse enabling a multitude of reactions to be run in water. Its versatility has been noted not only in terms of its usage in chemocatalysis, but also for its prominent role in biocatalyzed processes, which, when both are combined into sequential reactions in water, make it almost indispensable for applications to chemoenzymatic catalysis.
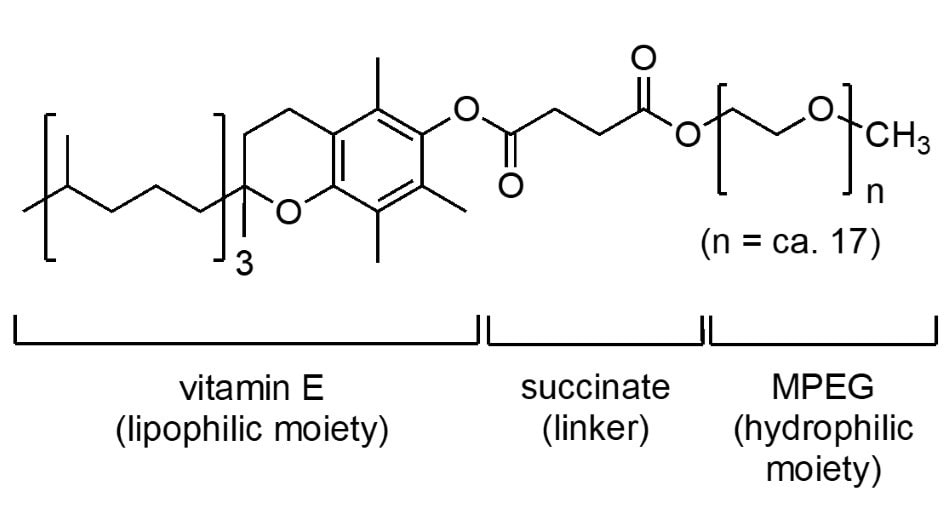
Figure 2.The structure of TPGS-750-M which is also known as DL-α-Tocopherol methoxypolyethylene glycol succinate.
Nok (SPGS-550-M)
This 3rd generation surfactant (Figure 3) is constructed from β-sitosterol and MPEG-550, also attached (as with TPGS-750-M) via a succinate linker. It is another general-purpose alternative to vitamin E-based TPGS-750-M (and Savie; vide infra). Unlike most commercial sources of vitamin E used in the manufacture of these surfactants, the price of plant-derived β-sitosterol is not reliant on petroleum, and therefore is typically far less expensive, thereby potentially providing a more cost-effective means of enabling micellar catalysis. Nok has been shown, on occasion, to be an effective alternative to TPGS-750-M in a number of reactions using its nanorod-shaped micelles, rather than the spherical micellar arrays characteristic of the other amphiphiles described herein.
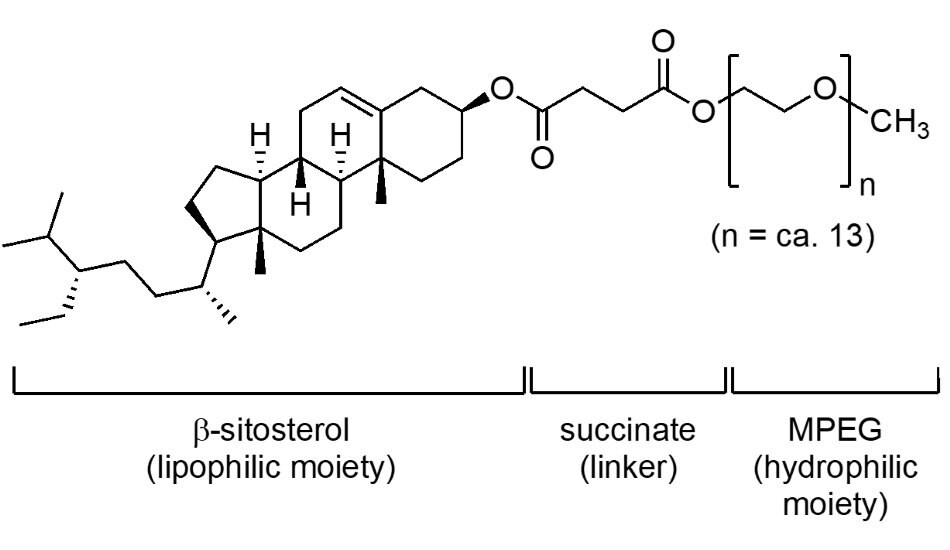
Figure 3.The structure of SPGS-550-M also known as NOK.
Savie
Savie (Figure 4) is the latest surfactant being added to the list containing a vitamin E-based lipophilic portion. The MPEG-750 is replaced by polysarcosine (PSar, which is just a polymer of N-methylglycine) that, likewise, enables reactions to be run in water. These range from varying types of Pd-catalyzed transformations to those catalyzed by numerous enzymes, including 1-pot, multistep chemoenzymatic sequences. In comparison to PEGylated surfactants, the PSar section of Savie is known to be fully biodegradable. In water, Savie gives rise to more stable emulsions, thereby reducing the need for organic co-solvents, and leading to more rapid rates of conversions, and hence, higher isolated yields for a given reaction time. It’s powdery consistency (as opposed to the waxes characteristic of PEGylated surfactants, including TPGS-750-M) makes it easy to handle and dispense, and it dissolves in water within seconds.
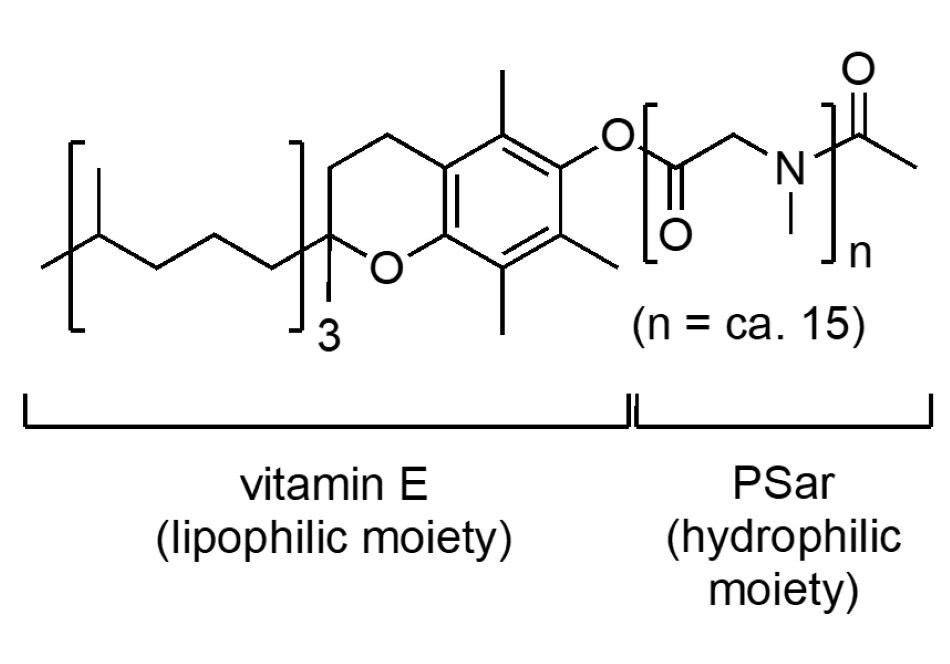
Figure 4.The structure of the surfactant Savie.
Coolade
Coolade (Figure 5) is a low-foaming, nonionic surfactant developed for use in gas-utilizing and/or -forming reactions. Surfactants, like soaps, tend to foam due to the hydrocarbon chains usually present in their makeup. With Coolade, however, made from grape-flavoring methyl anthranilate, the lack of such a structural feature leads to little-to-no frothing and hence, a simple solution to reactions that would otherwise require considerable headspace to accommodate gas usage and/or evolution. Applications of Coolade-derived micelles include Ni-catalyzed dehalogenation reactions, nitro group reductions, and the preparation of Pd nanoparticles with NaBH4.
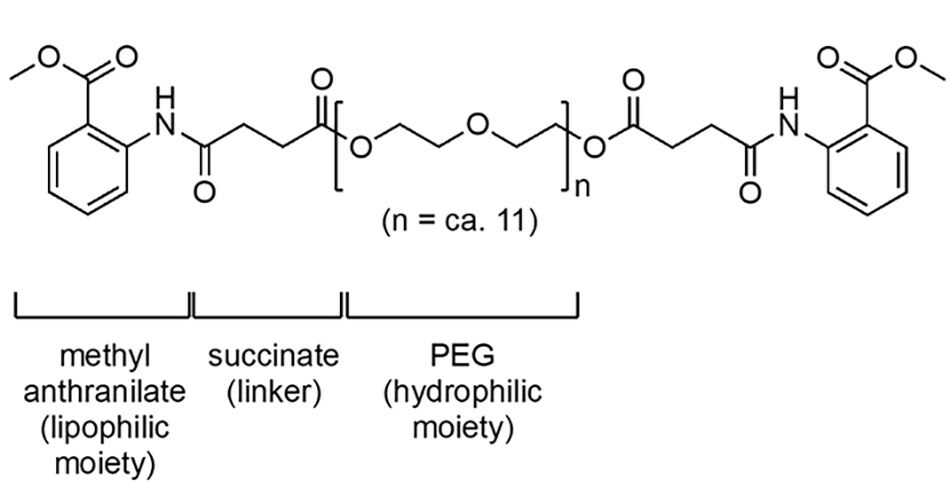
Figure 5.The structure of the surfactant Coolade.
MC-1
MC-1 (Figure 6) is a surfactant designed especially for enabling peptide coupling reactions in water. Its DMSO-like lipophilic core contains a polar sulfone moiety that houses polar amino acid coupling partners within the lipophilic interior of its self-assembled micelles. This simple change leads to greater rates of conversion compared to the purely hydrophobic micellar interiors formed by, e.g., TPGS-750-M.
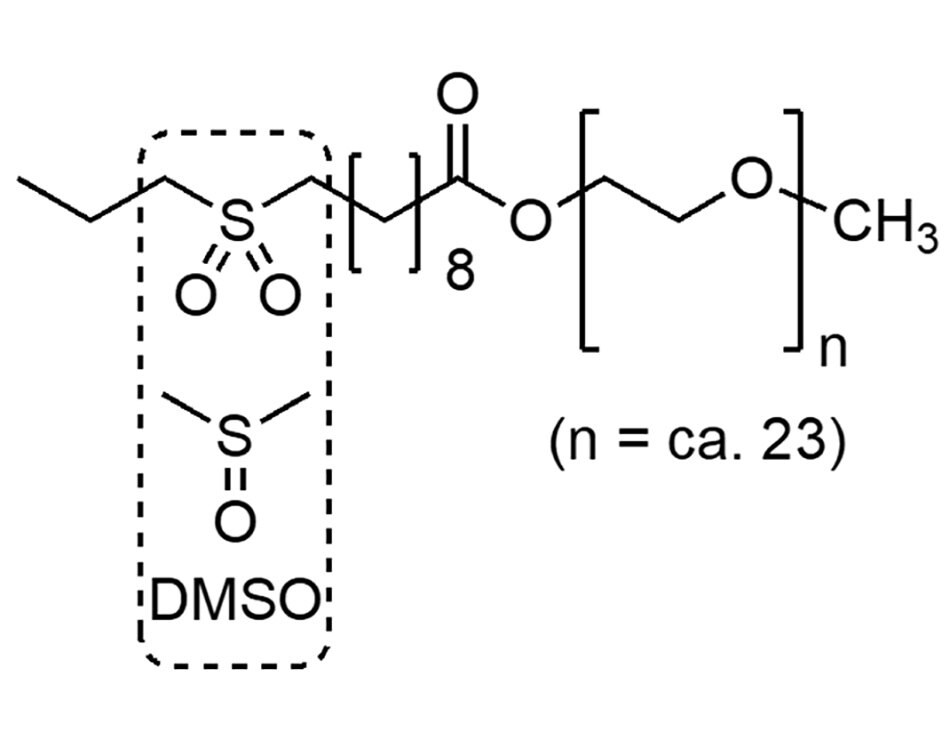
Figure 6.The structure of the surfactant MC-1.
References
Um weiterzulesen, melden Sie sich bitte an oder erstellen ein Konto.
Sie haben kein Konto?