pDADMAC Flocculation in Monoclonal Antibody Production Processes – Overcoming Regulatory Challenges with Quality Risk Management
Cell density titers used in the manufacturing of monoclonal antibodies (mAb) have increased dramatically over the past several years. Consequently, higher cell density can result in a bottleneck for subsequent purification of the mAb. The use of a flocculation agent such as a polycationic polymer polydiallyldimethylammonium chloride (pDADMAC), can improve clarification performance of these high density feedstreams.
Read more about
- What are flocculants and why are they used in downstream bioprocessing?
- Technical considerations when implementing flocculants
- Implementing flocculation technology
- Concerns and challenges with flocculant implementation
- Flocculant safety margin calculation
- Introducing a flocculant to your process can be considered a minor change
What are flocculants and why are they used in downstream bioprocessing?
Flocculation effectively removes excess cell debris from high-density cell culture, making the clarification and subsequent purification process more efficient 1. The charge of the flocculant, typically positive, also enables effective removal of negatively charged host cell proteins (HCP) and nucleic acid contaminants.
As indicated in the table below, there are a variety of flocculation agents available, such as pDADMAC and PEI, although not all are obtainable in a GMP grade suitable for bioprocessing. Flocculants should be distinguished from precipitation agents, such as caprylic acid or phosphoric acid, which can have similar effects on the particle size distribution and purity of the feed but via a different mechanism.
Flocculation as a clarification method is not new in bioprocessing or other process related industries. pDADMAC itself is used extensively in wastewater treatment as well as other precipitation agents and has a long history in microbial based bioprocesses, including in the production of GMP grade clinical material.
Technical considerations when implementing flocculants
The incorporation of flocculation technologies for biopharmaceuticals produced in mammalian cells has been slow due to technical challenges and perceived regulatory challenges (to be discussed below). However, many of these technical considerations can be mitigated within your process.
Flocculants are cationic
The flocculants currently being used within the biopharmaceutical industry are typically strongly cationic in nature (e.g. polyethylenimine (PEI), polydiallyldimethylammonium chloride (pDADMAC)). Most particulate contaminants, like cell debris, have a net negative charge at physiological pH 2. Likewise, other contaminants of concern like nucleic acids also bind to cationic polymers 3. For products of interest that are negatively charged, such as most viral vectors, polymer flocculation is not suitable as unacceptable yield losses will be observed.
Optimization of the flocculant dose
The dosing of the flocculant into the cell culture is critical to process impact and effectiveness. Care should be taken to determine the optimum dose for the cell culture conditions in order to ensure that the majority of the soluble flocculant is bound to the cells and other contaminants and to mitigate residual polymer concerns. Cell culture variability can be mitigated by performing a dosing study on a bioreactor sample just before harvest. Operator error with dosing levels can be addressed by effective process management and operational controls. It has been shown that with the dosing properly controlled, there is no impact on Protein A lifetime from the use of pDADMAC as a flocculant 4.
Ensure complete mixing of flocculants
Most flocculants are supplied as aqueous solutions, usually at 10% due to the highly viscous nature of the long chain polymers. The high viscosity can present mixing concerns, particularly during scale up. Typically, the flocculant addition is recommended over a ten-minute duration and the incubation time should be long enough to ensure complete mixing.
Implementing flocculation technology in biopharmaceutical processes
Implementation of flocculation technologies are hindered by a risk-averse biopharmaceutical industry, based on a historically conservative and stringent regulatory environment and a deeply ingrained rule-based compliance mindset. Chemistry Manufacturing and Control (CMC) changes are not only expensive but also typically result in prolonged timelines for regulatory approval and implementation. As a result, the pharmaceutical and biopharmaceutical industry have not been innovative, utilizing approaches and technologies that are decades old, with a high risk of negatively impacting drug safety and supply. What is not always recognized is that regulatory agencies, such as the FDA, EMA, WHO and ICH have been encouraging innovation and supporting a more efficient scientific risk assessment approach to change.
What does the regulatory landscape mean for the addition of a flocculation agent into an existing process? For process improvements after market approval, the manufacturer must assess the impact of the change on the quality, safety, and efficacy of their drug product. The quality of the drug substance is largely set prior to flocculation, harvest, and pooling, during the cell culture production process. Other quality attributes such as viral clearance, bioburden control, purity, and impurity levels, are either not impacted by flocculation or are improved 3. The highest regulatory stringency for a change occurs for a biopharmaceutical that has already been approved for market distribution.
In regulatory terms, the addition of a flocculation agent can be categorized as a “Change to a Fermentation or Cell Culture Process” and/or “Change to the Purification Process”, as cited in Appendix 2 of the WHO Annex 3: Guidelines on procedures and data requirements for changes to approved biotherapeutic products and in the EMA/FDA equivalents 5. Either category of change is scientifically justified to be a non-critical change with minimal potential to have an impact on the quality of the drug substance. Therefore, incorporating flocculation technologies fits the criteria for a minor risk, based on the WHO Annex 3 guideline 5. The risk categorization approach is aligned with the overall quality risk management (QRM) approach of other regulatory agencies, such as the FDA and EMA 14,18. A minor risk post-approval change categorization allows the drug manufacturer to implement the flocculant change prior to notification to regulatory agencies, which is also known as “Do and Tell”. Notification of regulators is typically conducted via the annual report, and regulatory approval of the change is not required.
Concerns and challenges with flocculant implementation
The main hinderances to the industrial application of flocculation technology for the purposes of cell and contaminant removal are:
- Residual polymer flocculant in the bulk drug substance or drug product
- Concerns regarding the toxicity of the flocculation agent
This information is a prerequisite for the drug manufacturer to move forward with a specific flocculation agent. For some types of aggregation approaches, such as precipitation with specific acids, salts or metal cations, limited toxicity concerns exist. However, a common issue regarding stability of the biologic drug substance under acidic or high salt conditions should be considered regarding process yields.
The ICH Q3A guidance, Impurities in New Drug Substances, can be used as an initial guide and gives thresholds for impurities as shown in Table 2 6. The initial concentrations for pDADMAC polymer are 0.01-0.05% 7. Since mAb therapeutic dosages are <2 g/day, the reporting threshold is listed at 0.05%, which is equivalent to 500 ppm. Therefore, the initial concentration of pDADMAC utilized during flocculation of mammalian cell culture is already at or below the threshold limit. For further evidence of in-process polymer removal and safety, studies have indicated that the residual concentrations of the pDADMAC polymer are well below 0.1 ppm following the downstream purification train, meaning that the residual polymer levels are 3-4 orders of magnitude below the reporting threshold limit 4.
pDADMAC Flocculant toxicity analysis
A footnote within the ICH Q3A impurity threshold limits (Attachment 1) mentions that "lower thresholds can be appropriate if the impurity is unusually toxic” 6. Based on internal in-vivo and in-vitro toxicity testing, this polymer is not toxic at the residual levels that would be present in the purified bulk drug substance, which is at ~0.1 ppm as determined by the limit of quantification of the analytical methods. Furthermore, no cytotoxicity or acute systemic toxicity was observed at this concentration in toxicological studies. In vivo irritant (intracutaneous injection) or hemolytic effects had only been detected at higher concentrations (~100 ppm or greater).
1 The amount of drug substance administered per day
2 Higher reporting thresholds should be scientifically justified
3 Lower thresholds can be appropriate if the impurity is unusually toxic
Flocculant safety margin calculation
What type of testing is the drug manufacturer required to conduct to show that the cationic polymer flocculant is efficiently removed during the manufacturing process? Does the flocculant level need to be a part of the mAb release specifications? Is the manufacturer required to conduct clearance testing during process development, characterization, or validation? Utilizing ICH Q6B (Specifications: test procedures & acceptance criteria for biotechnological/biological products) and ICH M7 (Assessment and control of DNA reactive (mutagenic) impurities in pharmaceuticals to limit potential carcinogenic risk), safety margins were calculated as follows:
- Sm = Le / Mm , where Mm is the measured maximum amount of the reagent per product dose in μg/dose and Le is the exposure limit for a potential safety concern (PSC) reagent in μg/dose. An estimated safety margin, Se, may be used during process development.
- When Sm is <1, either the process reagent should be changed, or the manufacturing process may be modified to ensure Sm ≥ 1 prior to use of the product in humans.
- If a Sm is low (1–5), it could become <1 for any given lot due to large lot‐to‐lot variability. In this case, regulatory agencies may require specification testing of the PSC reagent in each lot as a release assay. On the other hand, a high Sm (≥5) provides a reasonable justification that testing is not required.
Based on a daily dose of 300 milligrams of mAb for a 60 kg person: Sm = Le / Mm
For pDADMAC: Sm = 150 ug/dose/0.015 ug/dose (worst case); Sm = 10000
The reasonable parameters for the safety margin calculation are based on daily doses of 0.3 grams/day. In addition, with an unknown toxicity level, ICH M7 highlights ~120-150 ug/dose as the permitted daily exposure (PDE), although the actual PDE is likely higher. Finally, the limit of detection of innovative detection methods, such as qPCR and fluorescent labeling, are typically 50-100 ppb for the cationic polymers listed, meaning that the actual residual levels are slightly less. In the end, the safety margin is easily 3 orders of magnitude beyond the level needed for any testing. Therefore, no testing is required for incorporating these specific flocculants, with the available supplier and industry information.
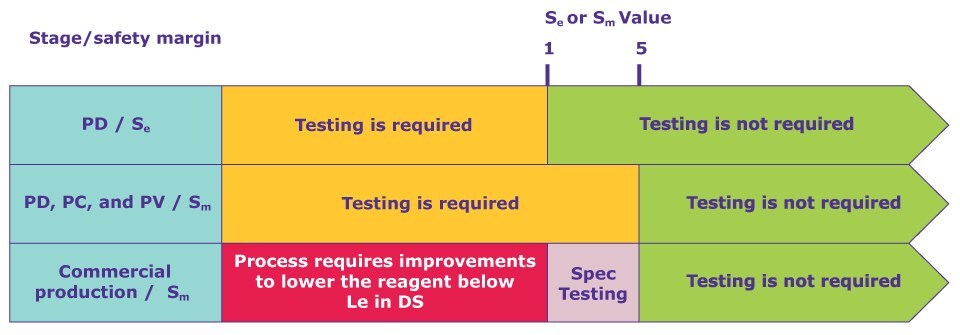
Figure 1. Safety margin as a criterion to determine the requirement of clearance testing for PSC reagents at different stages. Note: CPD is the abbreviation of the commercial process development. The parameter for determining Sm is measured from the drug substance.
Introducing a flocculant to your process is considered a minor change
From the information described in this article, mAb therapeutic manufacturers may implement the use of a flocculation agent as a minor change without the need for either clearance or specification release testing. This is the same minor risk category for incorporating a new Clarisolve® depth filter, designed to be used with the flocculation agent, with the stipulation that the updated clarification process does not impact the drug substance product quality. In the end, the drug manufacturer can follow a “Do and Tell” approach based on the minor change, meaning that they can go ahead and make the change without regulatory approval and notify the regulatory agency at a later date (e.g. annual report for the US FDA) 11,12,13.
References
Para seguir leyendo, inicie sesión o cree una cuenta.
¿No tiene una cuenta?