HPLC-UV Analysis of Native monoclonal antibody (mAb) and antibody-drug conjugate (ADC) on Widepore Silica Monolith Column Under High Flow Rate Conditions
Gisela Jung, R&D Scientist, Benjamin Peters, R&D Principal Scientist, Cory Muraco, Biomolecule Workflows Product Manager, Hillel Brandes, Analytical Technology Specialist
Section Overview
Introduction
Monolithic silica columns for HPLC offer low backpressure due to their high porosity (> 80%). This aspect leads to the possibility of using relatively high flow rates, resulting in reduced analysis time.
In this work, the influence of different parameters like flow rate, temperature, and solvent additives is shown for the determination of intact universal antibody standard human (Figures 2-13) and antibody-drug conjugate (ADC) mimic (Figures 14-15) on a Chromolith® WP 300 RP-18 column.
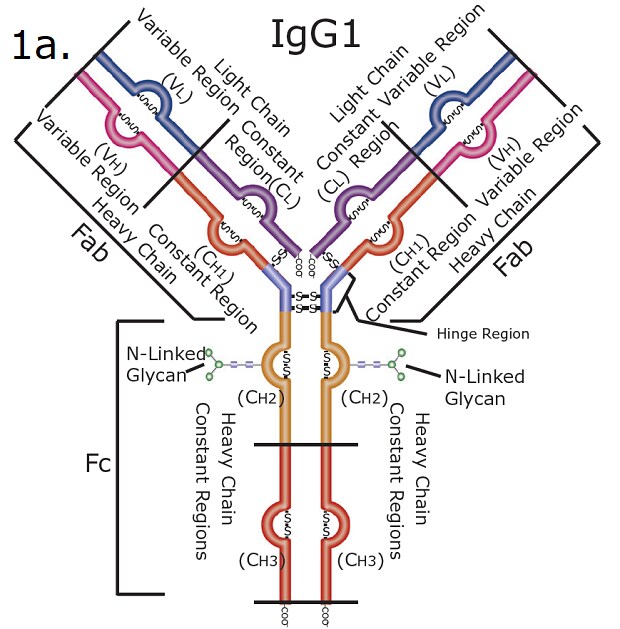
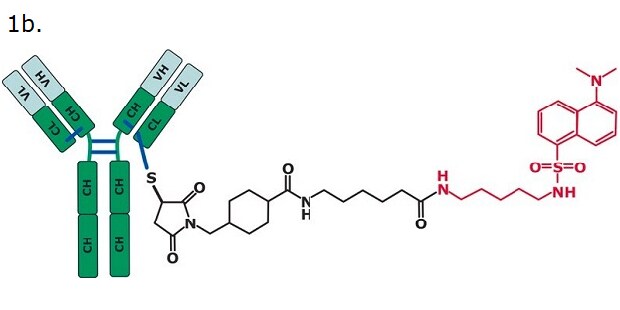
Figure 1. SILu™Lite SigmaMAb Universal Antibody Standard human (1a) and SigmaMAb Antibody-Drug Conjugate (ADC) Mimic (1b).
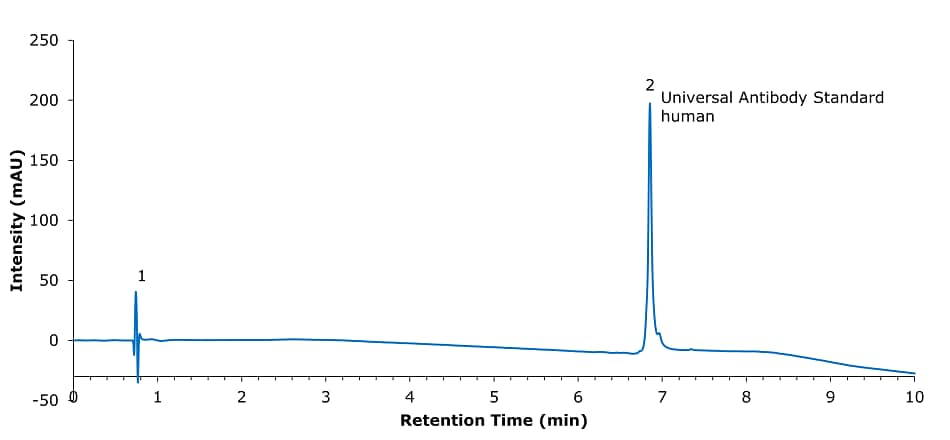
Figure 2.Analysis of SILu™Lite SigmaMAb Universal Antibody Standard human on a Chromolith® WP 300 RP-18 (100 x 2 mm I.D.).
Analysis of Universal Antibody Standard Human at Different Temperatures
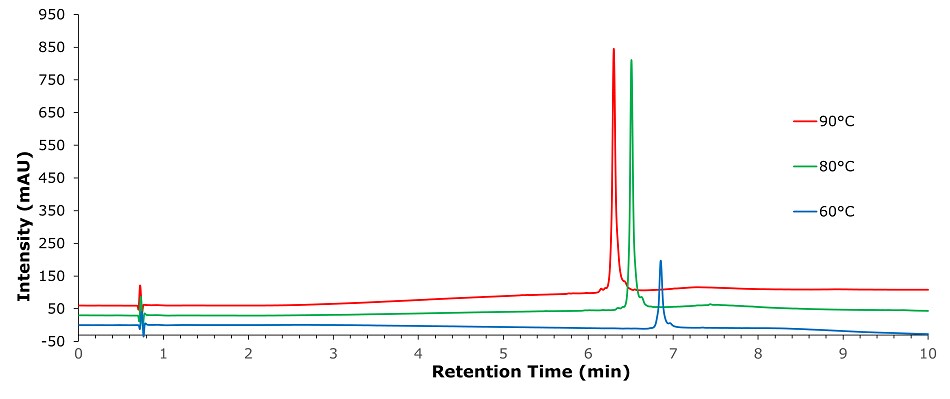
Figure 3.Analysis of SILu™Lite SigmaMAb Universal Antibody Standard human in matrix on a Chromolith® WP 300 RP-18 (100 x 2 mm I.D.) column at different temperatures
Analysis of Universal Antibody Standard Human with Different Solvent Additives on a Chromolith® WP 300 RP-18 Column
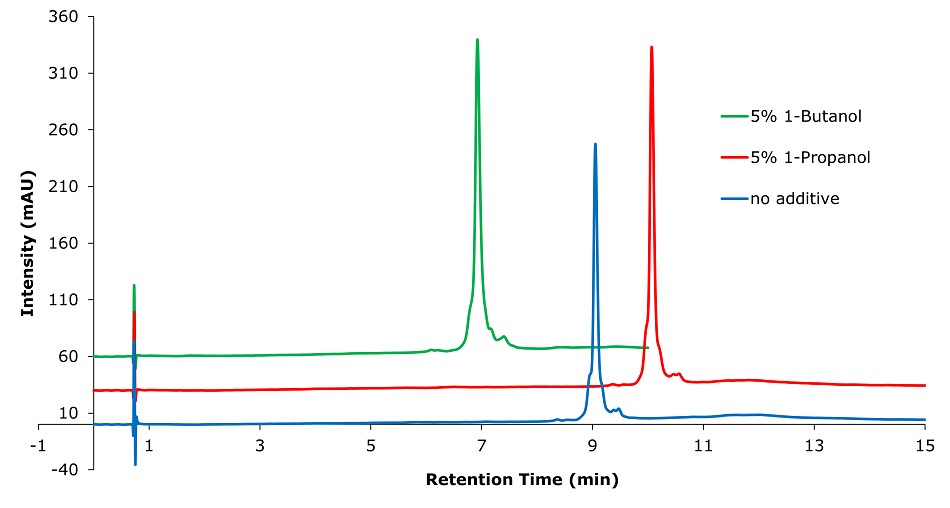
Figure 4.Analysis of SILu™Lite SigmaMAb Universal Antibody Standard human in matrix on a Chromolith® WP 300 RP-18 (100 x 2 mm I.D.) column with different solvent additives. Method: Temp. 60 °C, Mobile phase: A: 1-Propanol or 1-Butanol; B: Water 0.1% (v/v) TFA, C: Acetonitrile 0.1% (v/v) TFA; Gradient: 0 min A:5% B:70% C:25%, 0.1 min A:5% B:70% C:25%, 10 min A:5% B:60% C:35%.
Analysis of Universal Antibody Standard Human with Different Solvent Additives on a BIOshell™ A400 Protein C18 Column
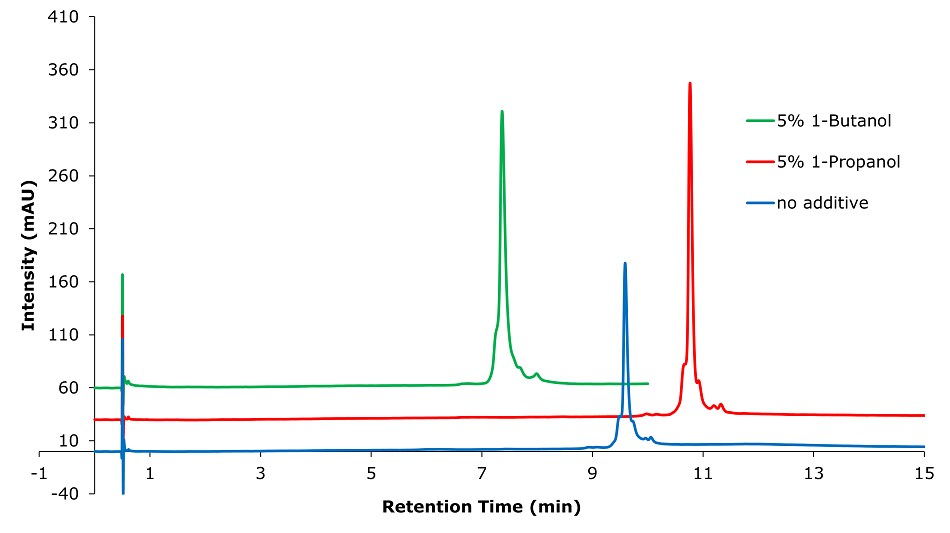
Figure 5.Analysis of SILu™Lite SigmaMAb Universal Antibody Standard human in matrix on a BIOshell™ A400 Protein C18, 3.4 µm (100 x 2.1 mm I.D.) column with different solvent additives. Method: Temp. 60 °C, Mobile phase: A: 1-Propanol or 1-Butanol; B: Water 0.1% (v/v) TFA, C: Acetonitrile 0.1% (v/v) TFA; Gradient: 0 min A:5% B:70% C:25%, 0.1 min A:5% B:70% C:25%, 10 min A:5% B:60% C:35%.
Analysis of Universal Antibody Standard Human with Premixed Mobile Phase Compared to Mobile Phase Mixed by Instrument Pump on a Chromolith® WP 300 RP-18 Column
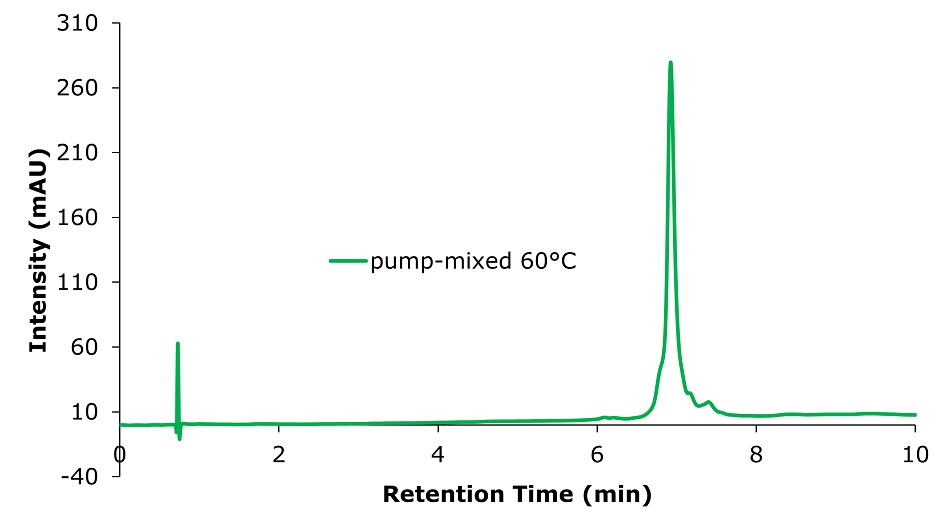
Figure 6.Analysis of SILu™Lite SigmaMAb Universal Antibody Standard human on a Chromolith® WP 300 RP-18 (100 x 2 mm I.D.) column with mobile phase mixed by instrument pump. Method: Temp. 60 °C, Mobile phase: A: Water 0.1 % TFA; B: Acetonitrile 0.1% TFA, C: 1-Butanol; Gradient: 0 min A:70% B:25% C:5%, 0.1 min A:70% B:35% C:5%, 10 min A:60% B:35% C:5%.
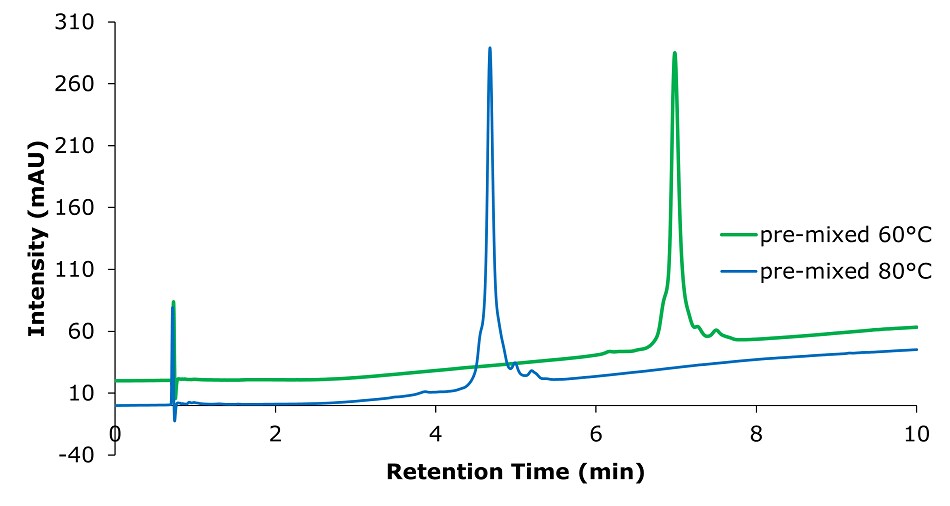
Figure 7.Analysis of SILu™Lite SigmaMAb Universal Antibody Standard human on a Chromolith® WP 300 RP-18 (100 x 2 mm I.D.) column with pre-mixed mobile phase at two different temperatures. Mobile phase: A: Acetonitrile/ 1-Butanol/ Water 25/5/70 0.1% (v/v) TFA, B: Acetonitrile/ 1-Butanol/ Water 35/5/60 0.1% (v/v) TFA, Gradient: 0 min A:100% B:0%, 0.1 min A:100% B:0%, 10 min A:0% B:100%.
Analysis of Universal Antibody Standard Human with Different Flow Rates on a Chromolith® WP 300 RP-18 Column
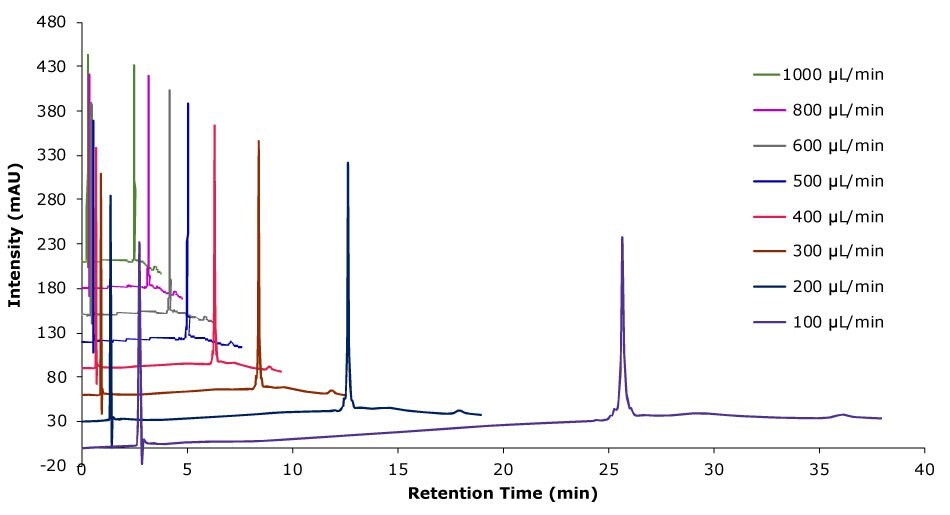
Figure 8.Analysis of SILu™Lite SigmaMAb Universal Antibody Standard human on a Chromolith® WP 300 RP-18 (100 x 2 mm I.D.) column with different flow rates. Method: Temp. 60 °C, Mobile phase: A: Water 0.1 % (v/v) TFA, B: Acetonitrile 0.1 % (v/v) TFA; Gradient: 20% B to 60% B, adjustment see table.
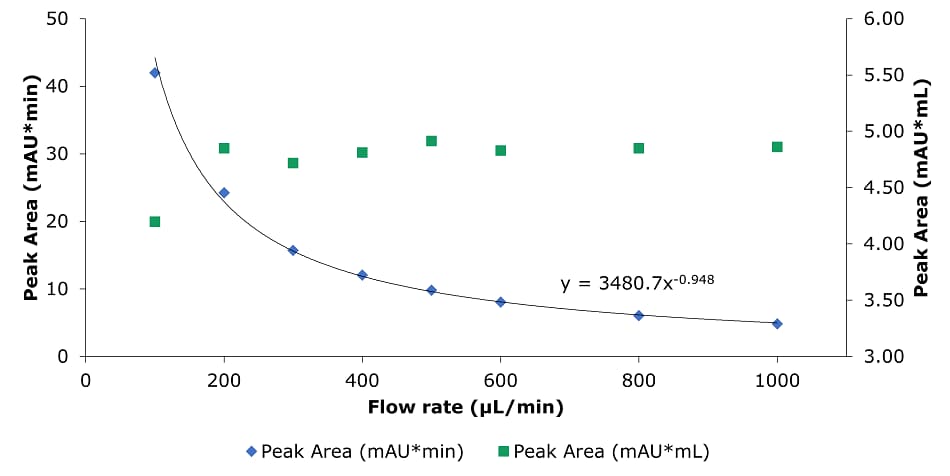
Figure 9.Analysis of SILu™Lite SigmaMAb Universal Antibody Standard human on a Chromolith® WP 300 RP-18 (100 x 2 mm I.D.) column. Peak area as a function of flow rate.
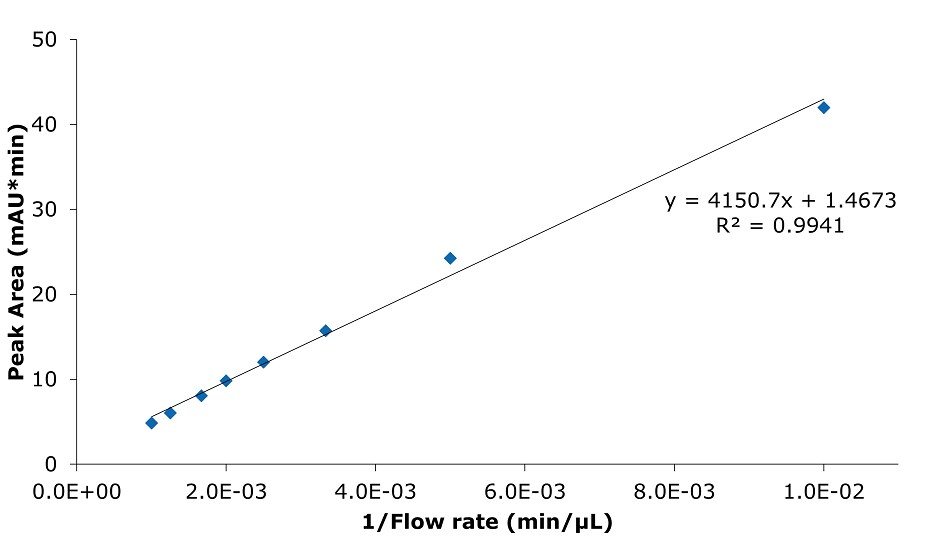
Figure 10.Analysis of SILu™Lite SigmaMAb Universal Antibody Standard human on a Chromolith® WP 300 RP-18 (100 x 2 mm I.D.) column. Peak area as a function of 1/flow rate.
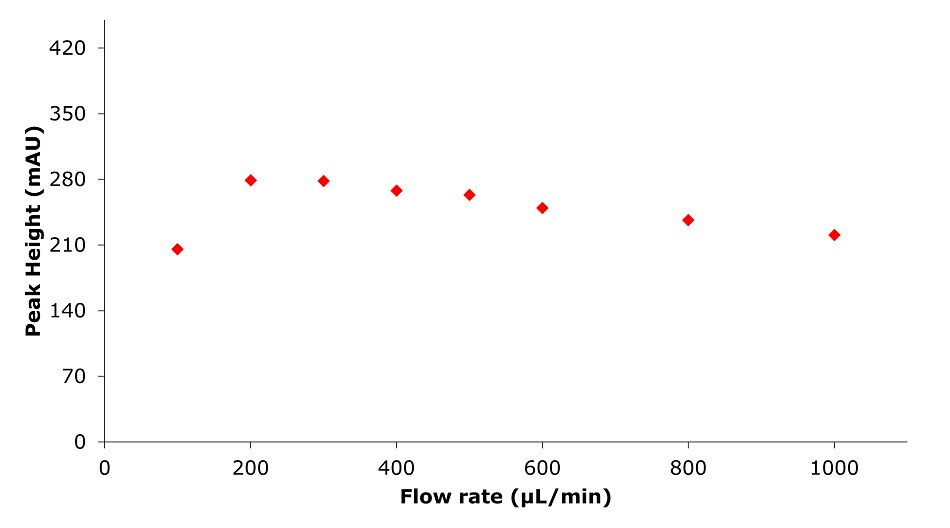
Figure 11.Analysis of SILu™Lite SigmaMAb Universal Antibody Standard human on a Chromolith® WP 300 RP-18 (100 x 2 mm I.D.) column. Peak height as function of flow rate.
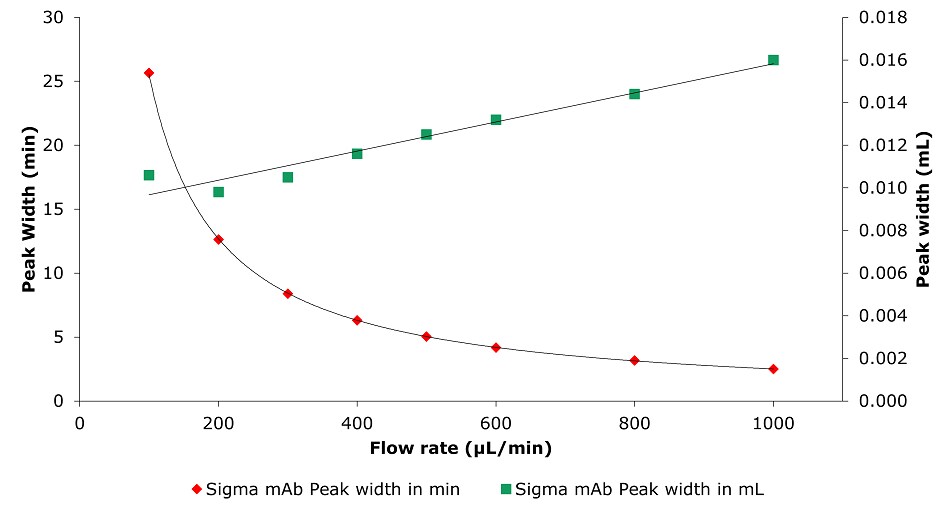
Figure 12.Analysis of SILu™Lite SigmaMAb Universal Antibody Standard human on a Chromolith® WP 300 RP-18 (100 x 2 mm I.D.) column. Peak width as function of flow rate.
Analysis of ADC Mimic at Different Temperatures and with Additive

Figure 13.Analysis of SILu™Lite ADC Mimic on a Chromolith® WP 300 RP-18 (100 x 2 mm I.D.) column at different temperatures and solvent additive.
Analysis of ADC Mimic with Different Flow Rates on a Chromolith® WP 300 RP-18 Column
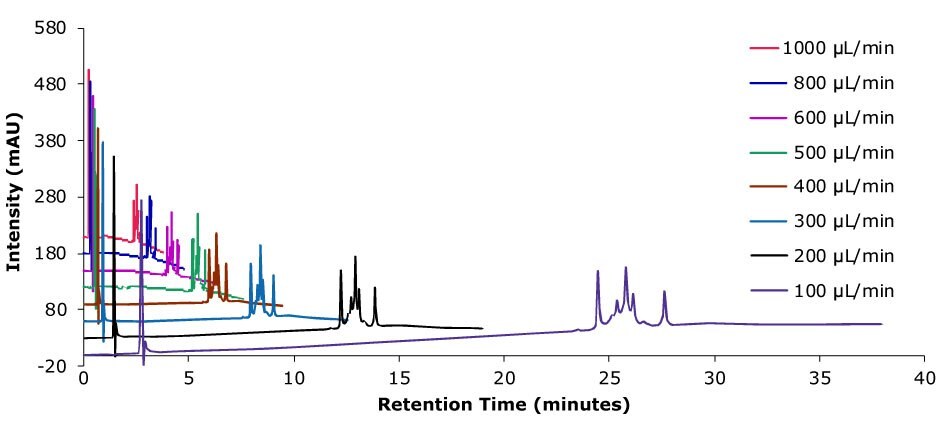
Figure 14.Analysis of SILu™Lite ADC Mimic on a Chromolith® WP 300 RP-18 (100 x 2 mm I.D.) column with different flow rates. Method: Temp. 60 °C, Mobile phase: A: Water 0.1 % (v/v) TFA, B: Acetonitrile 0.1 % (v/v) TFA; Gradient: 20% B to 60% B, adjustment see table.
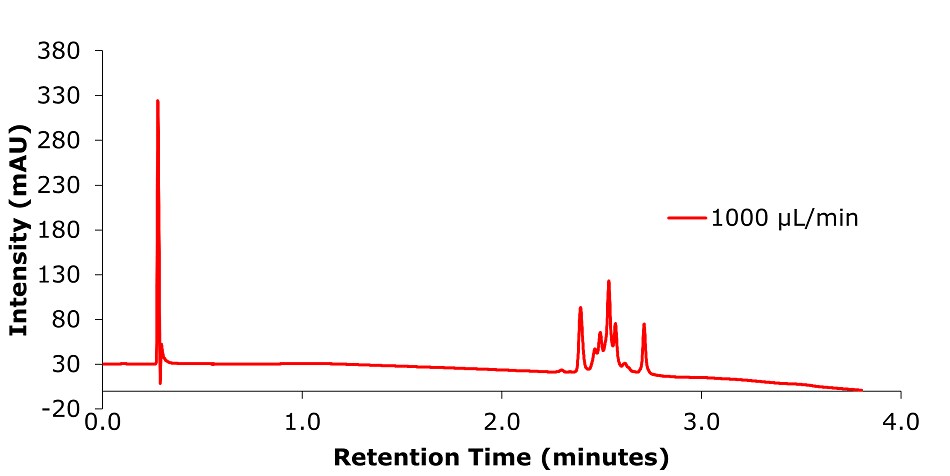
Figure 15.Analysis of SILu™Lite ADC Mimic on a Chromolith® WP 300 RP-18 (100 x 2 mm I.D.) column at 1 mL/min.
Conclusion
Within this work, it could be shown that intact SILu™Lite SigmaMAb Universal Antibody Standard human and SigmaMAb Antibody-Drug Conjugate (ADC) Mimic could be analyzed on silica monolithic HPLC columns under various conditions.
A significant increase in peak area with temperature change from 60°C to 80°C was observed as well as with the addition of solvent additives (5% 1-Propanol or 5% 1-Butanol). The combination of 60°C with solvent additive leads to the same result as increasing the temperature to 80°C, which can be beneficial as high temperature can lead to high-temperature artifact formation. Elevating the temperature to 90°C did not show a further improvement; also, there was no significant difference between pump-mixed and pre-mixed mobile phases. Additionally, it was found that the effect of adding 1-Butanol compared to 1-Propanol leads to a different retention behavior. 1-Butanol acts as a stronger eluting solvent, leading to shorter retention time compared to acetonitrile. 1-Propanol is known to have a high affinity for surface C18 chains, which seems to keep the C18 chains completely extended1 even under high water content environments in the mobile phase, leading to an increased retention time.
Higher flow rates lead to a tremendous time saving with only minor impact on column backpressure of the silica monolithic HPLC column, which can be beneficial, especially for biomolecules, as high pressure can affect conformational changes.2 Analyzing the data related to time, peak area, and peak width are decreasing with higher flow rate. Related to volume peak area is relatively stable, which is expected for a constant concentration, and peak width is increasing due to poor mass transfer kinetics of the biomolecule. Peak height is nearly constant, decreasing only slightly with increasing flow rate.
The described effects for peak area, peak height, and peak width for increased flow rates are related to the UV detection physical principle.3,4
Find more information on Biopharmaceutical Chracterization.
REFERENCES
Para seguir leyendo, inicie sesión o cree una cuenta.
¿No tiene una cuenta?