Bioburden Control in Bioprocessing
Bioburden control is key to successful drug manufacturing, and a contamination event upstream or downstream in your process can result in major disruptions and costs. Thinking holistically about contamination control, identifying critical points in the process, and mitigation actions to minimize contamination risk, is the foundation of a contamination control strategy and successful processing.
How is Bioburden Control Different in Upstream and Downstream Processing
Upstream Processes
In upstream processing, bioburden control focuses on the sourcing and characterization of raw materials to prevent the introduction of adventitious agents into cell culture operations. These processes are typically aseptic, and cell culture media is usually processed through filters that remove bacteria and mycoplasma before introducing the media to the process. Rapid testing methods help assure contaminated preharvest material is not transferred into downstream processes.1
Downstream Processes
Most downstream operations for monoclonal antibody therapies are not considered aseptic and operate as “low bioburden” or “bioburden-controlled”. Manufacturers typically set their own levels for bioburden. The BioPhorum’s Bioburden Working Group reported that action levels are commonly set at 1 to 10 colony forming units per mL.2 Later in downstream processing, stringent aseptic control is required as the drug product is formulated, sterile filtered, and transferred into vials.
Three Approaches to Effective Bioburden Control: Assess, Mitigate, Monitor
Effective bioburden control strategies rely on three complementary approaches:
- Assessing bioburden
- Mitigating the risk of occurrence
- Monitoring on an ongoing basis to assure process control
Assess Your Bioburden Risks
Assessing the microbial profile of a process is the first step to evaluating risk; understanding which microbial contaminants are present and how many (ex: raw material testing) provides a baseline against which future operations can be benchmarked. Risk assessment tools can be used to critically evaluate all aspects of the upstream and downstream processes to determine the potential impact of contaminants.3 This methodical approach is based on recommendations from the International Council for Harmonisation document, ICH Q9.4 A comprehensive quality risk management (QRM) strategy relies on a scientific approach to both understand risks and prioritize mitigation actions.
The risk of contamination is high in upstream processes due to the nutrient-rich environments, which are ideal for microbial proliferation. Risks are also high at processing steps closer to the final product as there are fewer purification steps to remove microorganisms and a greater potential impact to product quality and patient safety. However, at intermediate processing steps the contamination risk is lower, as buffers and sanitizers are less hospitable to microbial growth. This differential risk sensitivity throughout biomanufacturing operations has been described as a “risk hammock” and can be helpful while thinking about risk mitigation (Figure 1).
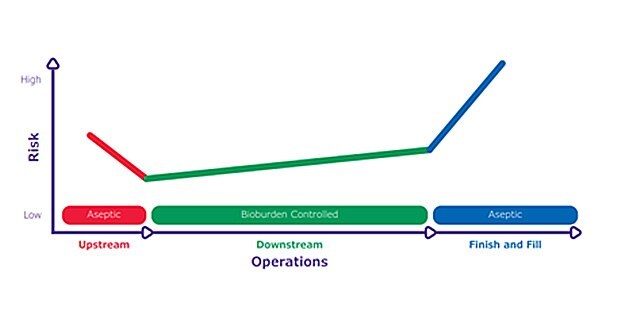
Figure 1.The “risk hammock” highlights differential risk sensitivity in biomanufacturing. Upstream operations are aseptic and have a high risk of contamination. Downstream operations control bioburden and the risk of contamination is lower. The finish and fill step is performed aseptically and any contamination presents the highest risk to patient safety.
There are many potential entry routes for microbial contaminants into a downstream process: improper cleaning or sanitization, risky aseptic connections, suboptimal system design, and lapses in aseptic technique.
Even though the risk of contamination is lower at intermediate processing steps, it is not zero. Some intermediate processing steps involve components that are reused multiple times, such as chromatography resins and tangential flow filtration devices. Typically, these components cannot be sterilized, and sanitization is performed to minimize bioburden. Unfortunately, packed chromatography columns offer an ideal environment for microbial growth and biofilm formation. The Protein A column is particularly problematic as it is the first purification step, loaded with nutrient-rich material that facilitates microbial growth. This, coupled with the fact that many effective sanitizing solutions can negatively impact resin performance, makes the Protein A capture step particularly challenging for microbial control.
Mitigate Bioburden Risks in Bioprocessing
Mitigating the risk of bioburden relies on the control of materials, facility cleaning and sanitization, containment, and removal of contaminants using filtration. Preventing microbial entry to biopharmaceutical production processes starts with the raw materials for production and careful consideration of the origin, supplier, and supplier’s quality management systems. In addition, the supplier’s characterization of the material and suggested quality level (see our M-Clarity™ Program) may impact handling and processing before use or even result in a change of supplier.
Sanitizers are particularly important in the intermediate processing steps where aseptic operations are not an option. Sanitization reduces but does not eliminate bioburden and can increase the levels of microbial cellular debris, such as bacterially-derived hydrophobic lipopolysaccharide molecules (endotoxins), following treatment. For this reason, it is often beneficial to minimize bioburden by containment and filtration, rather than relying solely on sanitization for microbial kill.
Containment such as single-use assemblies and sterilized fluid transfer systems link disposable fluid paths between process steps, minimize microbial ingress and, because they are available pre-sterilized, should not contribute to bioburden levels. Similarly, closed sampling technologies minimize the risk of introducing contaminants into the product flow path. Filtration is an excellent option for bioburden control, but before implementation, process design and operational best practices should be established and optimized. Any single-use system or filter should be compatible with the process fluid and meet performance needs in terms of bioburden control, processing efficiency, and cost.
Filtration Considerations for Bioburden Control
Upstream operations: Membrane filters with 0.1 µm or 0.2 µm membrane pore size are typically used to process cell culture media. Filter selection is based on processing costs, manufacturer experience, and level of risk tolerance.
Intermediate downstream operations: Sterilizing-grade or bioburden reduction filters can be used. Understanding the specific needs of the process based upon the manufacturer’s bioburden profile and the results of their risk assessment should guide filter selection.
Final sterile filtration: A sterilizing grade filter for liquid process streams contains a membrane with a rated pore size of 0.2 µm or smaller, validated based on the ASTM F838 bacterial retention standard. In addition, biomanufacturers must perform filter validation studies to confirm sterilizing grade performance of the filter and compatibility with their fluid stream under process conditions.
Maintain Control by Bioburden Monitoring
Monitoring helps determine typical bioburden levels in downstream operations and, when the threshold is exceeded, can trigger an investigation. Understanding the type of contaminant may help identify the source of the problem and help establish corrective actions.
Balancing bioburden safety with sampling and testing is always a challenge and relies on a risk analysis to identify the appropriate strategy for each process. Bioburden excursions are a real risk to the manufacturing process—they can be disruptive and costly. Effective control relies on process understanding and a comprehensive mitigation plan to assure safe drug production.
Download the Bioburden and Aseptic Control eBook
Our eBook “Mission Control: Strategies for Effective Bioburden and Aseptic Control” was created to address various aspects of bioburden control.
Topics include:
- A more detailed look at bioburden control in downstream processing
- How to select filters based on your bioburden challenge
- Considerations for maximizing the efficiency of sterilizing-grade filtration
- Industry trends in sterile filtration
- How quality by design approaches minimize risk
Fill out the form below to get the eBook delivered straight to your inbox.
References
Para seguir leyendo, inicie sesión o cree una cuenta.
¿No tiene una cuenta?