Simplifying Antibody-Drug Conjugate Payload Synthesis Using Payload Intermediates
With their ability to target specific cells and minimize off-target toxicity, antibody drug conjugates (ADCs) are being leveraged to treat serious diseases including cancer. To date, eleven ADCs have been approved and hundreds are in development. These complex biologics consist of a targeting antibody, a cytotoxic payload, and a linker. The linker attaches the payload to the antibody and is designed to be stable in circulation and often releases the payload once the ADC is inside the targeted cells.
Payloads Used in ADC Development
While more than forty natural product classes have been used as payloads, most of the ADCs that have entered the clinic incorporate derivatives of two natural products: dolastatin 10 and maytansine (Figure 1). Along with pyrrolobenzodiazepine (PBD) dimers, these payloads account for 75% of ADC payloads.
Dolastatins
Dolastatin 10 (Figure 1) is a tubulin inhibitor isolated from the Indian Ocean sea hare (Dolabella auriculari) and the potent derivatives MMAE and MMAF are included in five commercially available ADCs.
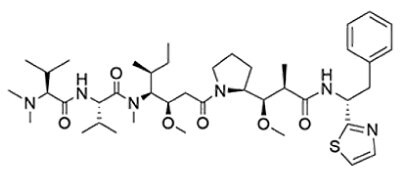
Figure 1.Chemical structures of dolastatin 10
Maytansines
Maytansine (Figure 2), derived from an east African shrub (Maytenus ovatus) is also a tubulin inhibitor and derivatives of maytansines are used in two commercial ADCs.
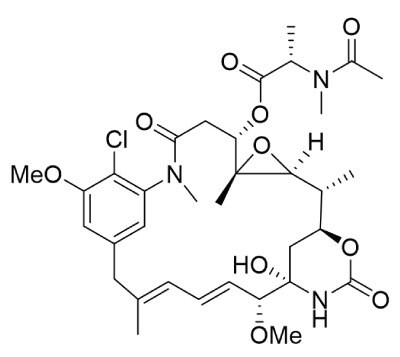
Figure 2.Chemical structure of maytansines.
Pyrrolobenzodiazepine (PBD) Dimers
PBD dimers (Figure 3) are notably potent, exhibiting higher potency than maytasines and dolastatins. They form rapid cytotoxic DNA cross-links in the minor groove of DNA and have been shown to have anti-tumor effects in both solid tumors and hematologic cancers. PBD dimers induce toxicity in varying tumor cell types, including those with low antigen expression or are multidrug resistant. The first PBD-based ADC was approved for the treatment of relapsed/refractory large B-cell lymphoma in April 2021.
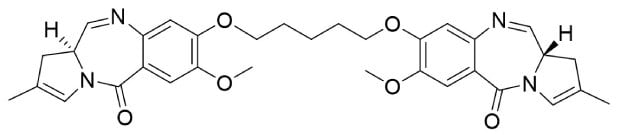
Figure 3.Chemical structures of PBD dimers.
Historic Pathway for Developing ADC Payloads
For developers of ADCs, gaining access to the desired payload can be challenging as their synthesis is more complex compared to most other small molecules, requiring a multi-step, labor-intensive process. For research purposes, some commonly used payloads can be purchased off-the-shelf from a number of different suppliers. Clinical development, however, requires a secure cGMP supply; in this case, there is a potential to license the compound. This can require a fair amount of time for negotiation and costs and typically includes milestones and royalty payments. A further challenge is that the specific structure might not be available, as licensing ultimately depends on the willingness of the originator.
Novel compounds are typically synthesized from scratch either in-house or in conjunction with a contract development and manufacturing organization (CDMO). The synthetic route must be established, along with a reliable and robust process for production and testing methods to identify possible impurities. The impurity profile then needs to be managed throughout development and manufacturing.
Simplifying ADC Synthesis Using ADC Payload Intermediates
Use of a cGMP-quality payload intermediate as a starting point for payload synthesis enables a more efficient process for research and process development chemists and chemistry, and manufacturing controls (CMC) teams. These intermediate compounds are the core of the active ingredients of dolastatin derivatives, maytansines, and PBD dimers. By using these cores as the starting point, what may have required a 15–20 step chemical synthesis will become three to four steps.
MAYCore™ Intermediate
The MAYCore™ intermediate (Figure 4) can be used to generate any of the maytasine-based payloads currently under investigation. The synthesis of maytansine-based payloads is prone to impurities such as D-ala-MERI so we focused on controlling the levels of impurities during synthesis.
The MAYCore™ intermediate has both reduced levels of impurities during the synthesis process that is further removed downstream to below the limit of quantitation (Figure 5).

Figure 4.Chemical structure of MAYCore™ Intermediate.

Figure 5.HPLC chromatogram of the MAYCore™ intermediate.
DOLCore ™ Intermediate
The DOLCore™ intermediate shortens the synthesis of dolastatin payloads to four steps or fewer. Its structure includes two handles for coupling to the ADC; one handle is typically reacted with standard peptide coupling conditions after which the Boc protection can be removed with acid and then reacted with standard peptide coupling conditions (Figure 6). With DOLCore™, terminal modifications can be completed in four steps or fewer to achieve the final payload.
Any dolastatin derivative that has ever entered the clinic can be produced from this core compound. In addition, diastereomers of DOLCore intermediate are controlled in the synthesis, drastically minimizing downstream impurities and simplifying further synthesis.

Figure 6.Chemical structure of DOLCore™ Intermediate.
An assessment of all the stereogenic centers that can be an up or down and anything that has a reasonable chance of forming has been conducted and confirms the diastereomeric purity of the compounds. Potential impurities generated by theoretically labile stereocenters were independently synthesized; the chromatogram shown in Figure 7 shows a final product testing method for a typical lot of DOLCore™ intermediate. Retention time markers of the commonly observed byproducts or impurities are quite low and are shown in blue with their problematic groups circled in red. The typical observed purity is >99.5 A% with D-Val-DOL and des-Me-DOL impurities present, indicating a high-quality product with very high diastereoselectivity. With use of a core compound as a starting material, the stereogenic centers only need to be managed during the final few steps which reduces risk and increases simplicity.

Figure 7.DOLCore™ compound impurities are well controlled.
PBDCore ™ Intermediate
The PBDCore™ intermediate (Figure 8) allows for versatile late-stage modifications at the TBDPSO, NHBcc, OTBS, and ketone chemical groups. The protecting groups that can be selectively cleaved under mild conditions and a keytone group that serves as a functional handle for cross-coupling methodologies. PBDCore™ can be elaborated to common clinically relevant PBD Dimers.

Figure 8.Chemical structure of PBDCore™ Intermediate.
Use of ADC Payload Intermediates Saves Time and Cost
For researchers exploring a range of compounds, starting the synthetic route with a core compound accelerates the synthesis process, enabling many more compounds to be made and evaluated. From a CMC perspective, a shorter synthetic route increases speed to clinic because just a few steps must be performed and managed.
Contact us to learn more about our ADC payload intermediates and to accelerate drug development.
Para seguir leyendo, inicie sesión o cree una cuenta.
¿No tiene una cuenta?