Piroxicam Capsules-Assay and Organic Impurities Following United States Pharmacopoeia Pending Forum Method
Dr Sanjay Poman,
Mumbai Application Laboratory
India
Introduction
Piroxicam (Figure 1) is an oxicam NSAID (non-steroidal anti-inflammatory drug) and is used to relieve the symptoms of painful inflammatory conditions like arthritis.1
A simple, precise, and sensitive reverse-phase high-performance liquid chromatography (RP-HPLC) gradient method (Table 1) using an Ascentis® Express C18 column was adopted for the assay and organic impurities analysis of Piroxicam capsules as part of method modernization from the USP-NF.2 The method was assessed in relation to USP general chapters <621>, <1225>, and <1226>.
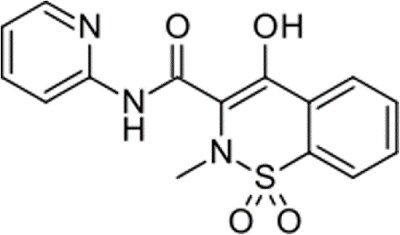
Figure 1.Chemical structure of Piroxicam
Piroxicam Capsules Assay And System Suitability Test
Experimental | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Experimental Conditions | |||||||||||||||||||||||||
| Column: | Ascentis®Express C18 100 x 4.6 mm I.D., 2.7µm (53827-U) | ||||||||||||||||||||||||
| Mobile Phase: | [A] Formic acid: ammonium hydroxide: water (0.1:0.35:100 v/v/v); [B] Acetonitrile | ||||||||||||||||||||||||
| Gradient: |
| ||||||||||||||||||||||||
| Flow rate: | 1 mL/min | ||||||||||||||||||||||||
| Pressure drop: | 167 bar (2421 psi) | ||||||||||||||||||||||||
| Column temp.: | 30° C | ||||||||||||||||||||||||
| Autosampler temp: | 4° C | ||||||||||||||||||||||||
| Detectior: | UV @ 254 nm (analytical flow cell, 10 µL) | ||||||||||||||||||||||||
| Injection: | 5 µL | ||||||||||||||||||||||||
| Samples | |||||||||||||||||||||||||
| System suitability solution: | Organic Impurities: 1.0 mg/mL of Piroxicam RS and 10 μg/mL Piroxicam Related Compound B RS mobile phase | ||||||||||||||||||||||||
| Standard solutions: | Assay: Dissolve 0.1 mg/mL of Piroxicam RS in methanol Organic Impurities: 2 μg/mL of Piroxicam RS and 10 μg/mL of Piroxicam Related Compound A in methanol | ||||||||||||||||||||||||
| Sensitivity solution: | Organic impurities: 0.5 μg/mL of Piroxicam RS in methanol, sonicate if needed. | ||||||||||||||||||||||||
| Sample solutions: | Assay (0.1 mg/mL): Transfer a portion of the mixed content of capsules equivalent to 10 mg of piroxicam to a 100 mL volumetric flask, add about 80 ml of methanol, and swirl to disintegrate. Sonicate for 5 minutes and agitate by mechanical means for 30 minutes. Dilute with methanol to volume. Centrifuge to obtain a clear solution. Organic impurities (1 mg/mL): Transfer a portion of the mixed content of capsules equivalent to 20 mg of piroxicam in a 20 mL volumetric flask. Add 15 mL of methanol, and swirl to disintegrate. Sonicate for 5 minutes and agitate by mechanical means for 30 minutes. Dilute with methanol to volume. Centrifuge to obtain a clear solution. | ||||||||||||||||||||||||
Results
Assay
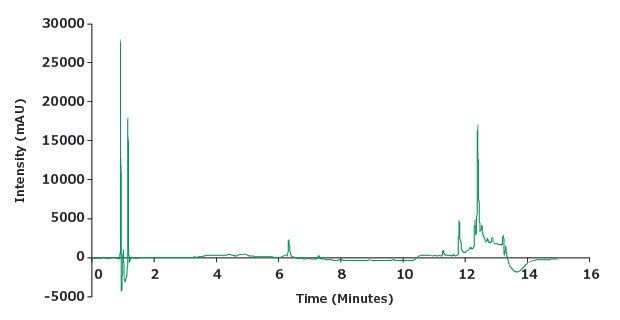
Figure 2.Chromatographic blank run.
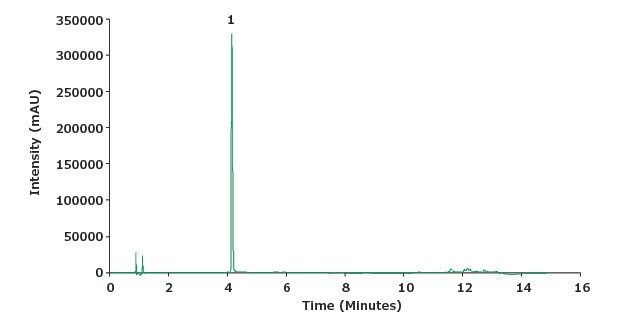
Figure 3.Chromatogram of a piroxicam standard solution (0.1 mg/mL).
Chromatographic Data for Standard Solution | ||||
|---|---|---|---|---|
| Peak | Compound | Retention Time (min) | Tailing Factor | Area |
| 1 | Piroxicam | 4.1 | 1.5 | 952666 |
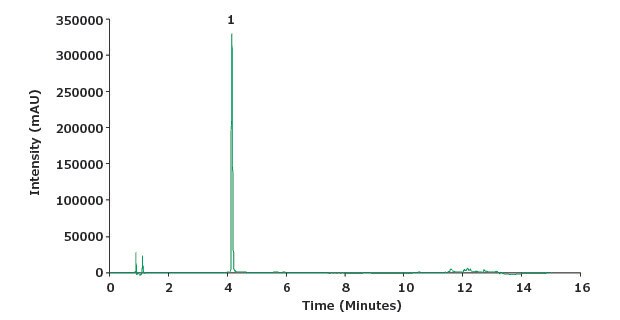
Figure 4.Chromatogram of piroxicam sample solution (0.1 mg/mL)
Chromatographic Data for Sample Solution | ||||
|---|---|---|---|---|
| Peak | Compound | Retention Time (min) | Tailing Factor | Area |
| 1 | Piroxicam | 4.2 | 1.4 | 950353 |
| Sample (0.1 mg/mL) | Area |
|---|---|
| STD 1 | 952621 |
| STD 2 | 938699 |
| STD 3 | 946489 |
| STD 4 | 947663 |
| STD 5 | 952666 |
| Mean | 947627.6 |
| Standard Deviation | 5125.17 |
| RSD (%) | 0.54 |
System Suitability Requirements | ||
|---|---|---|
| Component | USP Specification | Observed value |
| % RSD | NMT 2.5% | 0.54% |
| Tailing Factor | NMT 2.0 | 1.5 |
Organic Impurities Analysis of Piroxicam Capsules
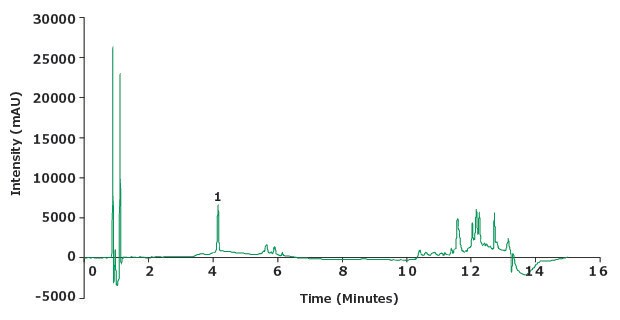
Figure 5.Chromatogram of the piroxicam sensitivity solution (0.5 µg/mL).
Chromatographic Data - Sensitivity Solution | ||||||
|---|---|---|---|---|---|---|
| Peak | Compound | Retention Time (min) | Tailing Factor | Area | S/N | USP Requirement S/N |
| 1 | Piroxicam 0.5 µg/ml | 4.1 | 1.02 | 16729 | 15.2 | NLT 10 |
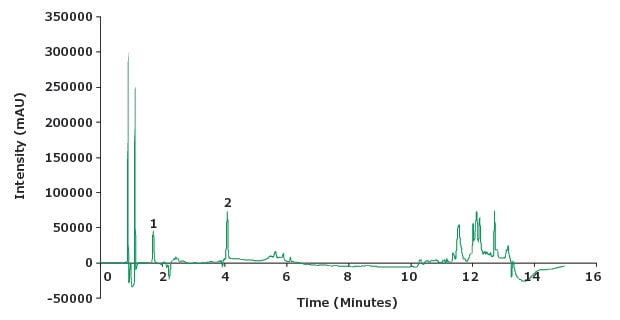
Figure 6.Chromatogram standard solution (2 µg/mL piroxicam and impurity A at 10 µg/mL).
Chromatographic Data – System Suitability Solution | ||||||
|---|---|---|---|---|---|---|
| Peak | Compound | Retention Time (min) | RRT | Tailing Factor | Resolution | Area |
| 1 | Impurity A | 1.7 | 0.41 | 1.02 | - | 13705 |
| 2 | Piroxicam | 4.1 | 1 | 1.36 | 31.2 | 19639 |

Figure 7.Chromatographic separation of standard solution organic impurities with 1 mg/mL piroxicam and impurity B at 10 µg/mL.
Chromatographic Data - Standard Solution Organic Impurities | ||||||
|---|---|---|---|---|---|---|
| Peak | Compound | Retention Time (min) | RRT | Tailing Factor | Resolution | Area |
| 1 | Piroxicam | 3.9 | - | 2.7 | - | 11088453 |
| 2 | Impurity B | 5.2 | 1.33 | 1.0 | 8.8 | 1115 |
System Suitability Requirements* | ||
|---|---|---|
| Component | USP Specification | Observed value |
| RRT Impurity A | 0.35 | 0.4 |
| RRT Impurity B | 1.1 | 1.3 |
| Resolution between Piroxicam & Impurity B | NLT 4.0 | 8.8 |
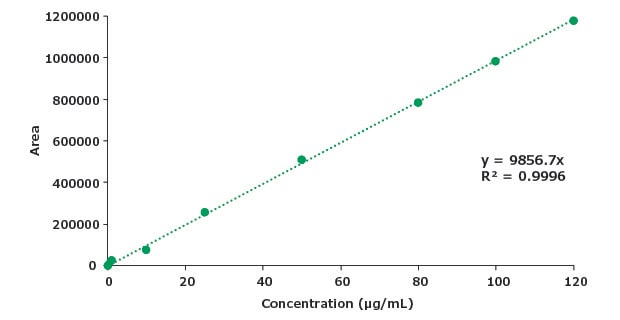
Figure 8.Calibration curve for piroxicam.
| Concentration (μg/mL) | Mean Area |
|---|---|
| 0.05 | 1215 |
| 0.5 | 12157 |
| 1 | 24314 |
| 10 | 75328 |
| 25 | 256425 |
| 50 | 509595 |
| 80 | 786270 |
| 100 | 982838 |
| 120 | 1179405 |
| STEYEX | 12771 |
| Slope | 9856 |
| LOD (μg/mL) | 4.27 |
| LOQ (μg/mL) | 12.9 |
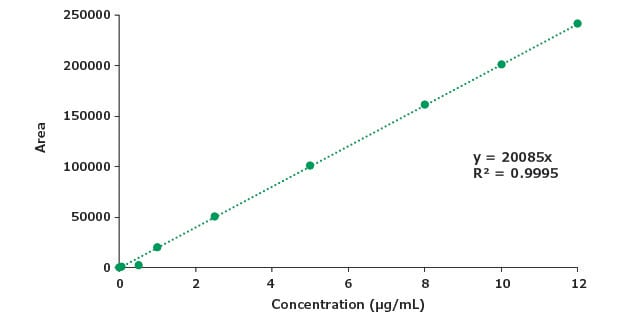
Figure 9.Calibration curve for impurity A.
| Concentration (μg/mL) | Mean Area |
|---|---|
| 0.005 | 100 |
| 0.05 | 1002 |
| 0.5 | 2002 |
| 1 | 20098 |
| 2.5 | 50245 |
| 5 | 100490 |
| 8 | 160784 |
| 10 | 200960 |
| 12 | 241152 |
| STEYEX | 2725 |
| Slope | 20085 |
| LOD (μg/mL) | 0.4 |
| LOQ (μg/mL) | 1.3 |
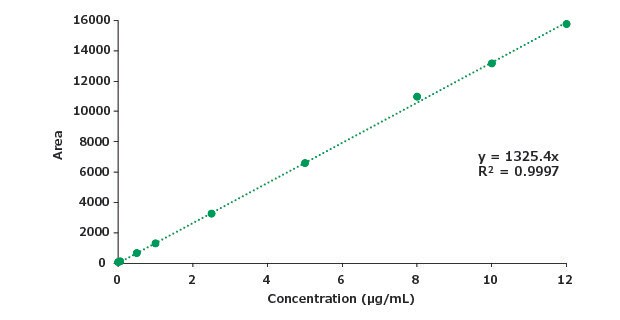
Figure 10.Calibration curve for impurity B.
| Concentration (μg/mL) | Mean Area |
|---|---|
| 0.005 | 60 |
| 0.05 | 111 |
| 0.5 | 657 |
| 1 | 1311 |
| 2.5 | 3280 |
| 5 | 6576 |
| 8 | 10960 |
| 10 | 13152 |
| 12 | 15782 |
| STEYX | 150.3 |
| Slope | 1325 |
| LOD (μg/mL) | 0.37 |
| LOQ (μg/mL) | 1.1 |
Conclusion
A sensitive, stability indicating gradient reversed-phase HPLC method has been developed for the quantitative estimation of piroxicam (assay) and organic impurities in a dosage form. System suitability criteria mentioned in the USP monograph for reference standard as well as the test solution of piroxicam dosage form were found to be in compliance with % RSD and tailing factor parameters for the assay. The developed method was found to be linear for piroxicam up to 120 µg/mL with LOD of 4.2 µg/mL and LOQ of 12.9 µg/mL. For the organic impurity A linearity was assessed up to 12 µg/mL with LOD and LOQ at 0.4 and 1.3 µg/mL respectively, for impurity B also up to 12 µg/mL with LOD and LOQ at 0.37 and 1.1 µg/mL respectively. The relative retention times for impurities A and B are in agreement with the RRT values mentioned in the USP monograph. %RSD for piroxicam and piroxicam related compound A standard solution was not determined. The method can be applied for related substances study of piroxicam dosage form using the Ascentis® Express C18 HPLC column.
Network error: Failed to fetch
References
To continue reading please sign in or create an account.
Don't Have An Account?