S2076
α-2,6-Sialyltransferase from Photobacterium damsela
recombinant, expressed in E. coli BL21, ≥5 units/mg protein
Synonym(s):
β-Galactoside α-2,6-sialyltransferase, CMP-N-Acetylneuraminate:β-D-galactosyl-1,4-N-acetyl-β-D-glucosamine α-2,6-N-acetylneuraminyltransferase
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
MDL number:
UNSPSC Code:
12352204
NACRES:
NA.54
Recommended Products
recombinant
expressed in E. coli BL21
Quality Level
form
lyophilized powder
specific activity
≥5 units/mg protein
mol wt
56.8 kDa
shipped in
dry ice
storage temp.
−20°C
General description
Human ST6Gal-I (β-galactoside α-2,6-sialyltransferase 1) is a member of the CAZy family GT29.
Application
α-2,6-Sialyltransferase from Photobacterium damsela has been used in resialylation and restoration of sialic acids (SAs) in HRT-18G cells.
Highly active α2-6 sialyltransferase has been used to prepare high levels of disialylated fragment crystals.
Biochem/physiol Actions
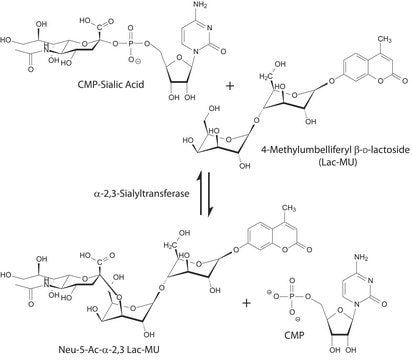
Sialyltransferase transfers Neu5Ac from CMP-Neu5Ac to the galactosyl terminus of acceptor molecules including glycoproteins, glycolipids, and oligosaccharides.
The terminal step of complex N-glycan biosynthesis is catalysed by α-2,6-sialyltransferase (STs). Bacterial α(2,6)-STs possesses broader acceptor substrate specificity when compared to eukaryotic α(2,6)-STs.
Unit Definition
One unit will catalyze the formation of 1 μmol Neu-5-Ac-α-2,6-LacMU from CMP-Neu-5-Ac and Lac-β−OMU per minute at 37 °C at pH 8.0.
Physical form
Supplied as a lyophilized powder containing Tris-HCl and NaCl.
Analysis Note
Enzymatic activity assays are performed in Tris-HCl buffer (100 mM, pH 8.0) containing CMP-Neu-5-Ac (1 mM) and Lac-β−OMU (1 mM) at 37 °C for 30 min and analyzed using HPLC with a fluorescence detector (excitation at 325 nm and emission at 372 nm).
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Enhanced Bacterial alpha (2, 6)-Sialyltransferase Reaction through an Inhibition of Its Inherent Sialidase Activity by Dephosphorylation of Cytidine-5'-Monophosphate
Kang JY, et al.
PLoS ONE, 10(7), e0133739-e0133739 (2015)
Nageswari Yarravarapu et al.
Bioconjugate chemistry, 33(5), 781-787 (2022-04-20)
Glycan binding often mediates extracellular macromolecular recognition events. Accurate characterization of these binding interactions can be difficult because of dissociation and scrambling that occur during purification and analysis steps. Use of photocrosslinking methods has been pursued to covalently capture glycan-dependent
Miyako Nakano et al.
Molecular & cellular proteomics : MCP, 10(11), M111-M111 (2011-08-24)
Resistance to tubulin-binding agents used in cancer is often multifactorial and can include changes in drug accumulation and modified expression of tubulin isotypes. Glycans on cell membrane proteins play important roles in many cellular processes such as recognition and apoptosis
High-quality production of human alpha-2, 6-sialyltransferase in Pichia pastoris requires control over N-terminal truncations by host-inherent protease activities
Ribitsch D, et al.
Microbial cell factories, 13, 138-138 (2014)
Tatsuya Kato et al.
Journal of bioscience and bioengineering, 113(6), 694-696 (2012-02-09)
Modified polyhedrin promoter (Ppolh) was designed by repeating burst sequences (BSs) and adopted to overexpress rat α2,6-sialyltransferase (ST6Gal I) in silkworm. Modified Ppolh of five BSs with VLF-1 coexpression yielded 2.9 U/ml ST6Gal I activity and 32.5 mU/mg specific activity
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service