C-12205
Human Umbilical Vein Endothelial Cells (HUVEC)
Pre-screened, 500,000 cryopreserved cells
Synonym(s):
HUVEC Cells, Human Endothelial Cells, Umbilical Vein Endothelial Cells
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
UNSPSC Code:
41106514
NACRES:
NA.81
Recommended Products
biological source
human umbilical cord vein
packaging
pkg of 500,000 cells
morphology
( endothelial)
technique(s)
cell culture | mammalian: suitable
shipped in
dry ice
storage temp.
−196°C
General description
Lot specific orders are not able to be placed through the web. Contact your local sales rep for more details.
Cell Line Origin
Vein
Application
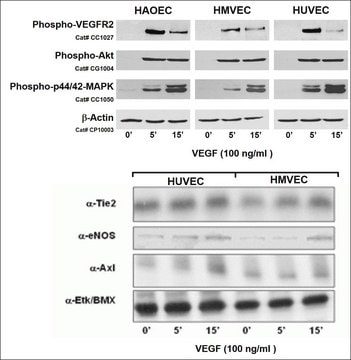
Primary Human Umbilical Vein Endothelial Cells (HUVEC) are isolated from the vein of the umbilical cord. They are commonly used for physiological and pharmacological investigations, such as macromolecule transport, blood coagulation, angiogenesis, and fibrinolysis. HUVEC are isolated from single donors in standard Endothelial Cell Growth Medium and provided in a cryopreserved format. The cells have been pre-screened for VEGF (vascular endothelial growth factor) response. VEGF is an important signaling protein involved in both vasculogenesis and angiogenesis. In vitro, VEGF has been shown to stimulate endothelial cell mitogenesis, cell migration, sprouting, and microvascular permeability.
Quality
Rigid quality control tests are performed for each lot of Endothelial Cells from large vessels. They are tested for cell morphology, adherence rate, and cell viability. Flow cytometric analyses for cell-type specific markers, e.g. von Willebrand Factor (vWF) and CD31, as well as Dil-Ac-LDL uptake assays are also carried out for each lot. Growth performance is tested through multiple passages up to 15 population doublings (PD) under culture conditions without antibiotics and antimycotics. In addition, all cells have been tested for the absence of HIV-1, HIV-2, HBV, HCV, HTLV-1, HTLV-2 and microbial contaminants (fungi, bacteria, and mycoplasma).
Warning
Although tested negative for HIV-1, HIV-2, HBV, HCV, HTLV-1 and HTLV-2, the cells – like all products of human origin – should be handled as potentially infectious. No test procedure can completely guarantee the absence of infectious agents.
Subculture Routine
Click here for more information.
Other Notes
Recommended Plating Density: 5000 - 10000 cells per cm2Passage After Thawing: P1Tested Markers: von Willebrand Factor (vWF) positive, CD31 positive, Dil-Ac-LDL uptake positiveGuaranteed population doublings: >15
Recommended products
Recommended Primary Cell Culture Media:Link
Disclaimer
RESEARCH USE ONLY. This product is regulated in France when intended to be used for scientific purposes, including for import and export activities (Article L 1211-1 paragraph 2 of the Public Health Code). The purchaser (i.e. enduser) is required to obtain an import authorization from the France Ministry of Research referred in the Article L1245-5-1 II. of Public Health Code. By ordering this product, you are confirming that you have obtained the proper import authorization.
Storage Class Code
12 - Non Combustible Liquids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service