A56655
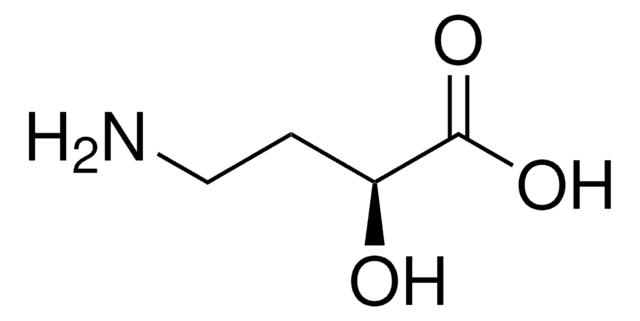
4-Amino-3-hydroxybutyric acid
98%
Synonym(s):
DL-γ-Amino-β-hydroxybutyric acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
H2NCH2CH(OH)CH2CO2H
CAS Number:
Molecular Weight:
119.12
EC Number:
MDL number:
UNSPSC Code:
12352106
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
powder or crystals
reaction suitability
reaction type: solution phase peptide synthesis
color
white to yellow
mp
223 °C (dec.) (lit.)
application(s)
peptide synthesis
SMILES string
NCC(O)CC(O)=O
InChI
1S/C4H9NO3/c5-2-3(6)1-4(7)8/h3,6H,1-2,5H2,(H,7,8)
InChI key
YQGDEPYYFWUPGO-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Izumi Yamamoto et al.
ACS chemical neuroscience, 3(9), 665-673 (2012-09-29)
Designing potent and subtype-selective ligands with therapeutic value requires knowledge about how endogenous ligands interact with their binding site. 4-Amino-3-hydroxybutanoic acid (GABOB) is an endogenous ligand found in the central nervous system in mammals. It is a metabolic product of
Zhenjun Du et al.
Chirality, 16(8), 516-519 (2004-08-04)
Rh(2)(4S-MEOX)(4) and ethereal solvent are the best catalytic system for the enantioselective intramolecular C-H insertion of N-(2-benzyloxyethyl)-N-(tert-butyl)diazoacetamide 2. The highest enantiomeric excess obtained was 91%. A new route for the asymmetric synthesis of gamma-amino-beta-hydroxybutyric acid (GABOB) has been developed.
M A Enero et al.
Clinical and experimental hypertension. Part A, Theory and practice, 10 Suppl 1, 331-337 (1988-01-01)
The cardiovascular effects of i.v. gamma-amino-beta-hydroxybutyric acid (GABOB) were investigated in rats anaesthetized with urethane. GABOB produced a dose-dependent hypotensive response. Treatment with GABA-A receptor antagonists prevented the GABOB response while the GABA stimulation by diazepam enhanced this response. The
G Bonardi et al.
Arzneimittel-Forschung, 31(11), 1910-1913 (1981-01-01)
1. Serum levels of DL-gamma-amino-beta-hydroxybutyric acid-1-14C (DL-GABOB-1-14C) in the rat (50 mg/kg) were quite similar after single i.v. and p.o. doses. Also the disappearance from the serum was similar with both administration routes. Within 6 days after p.o. treatment with
Stereoselective analysis of racemic psychotropic compounds by HPLC on chiral stationary phase.
D F Smith et al.
Psychopharmacology, 89(3), 392-393 (1986-01-01)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service