673110
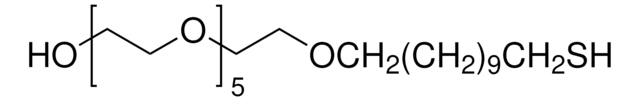
Triethylene glycol mono-11-mercaptoundecyl ether
95%
Synonym(s):
(11-Mercaptoundecyl)tri(ethylene glycol)
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
HS(CH2)11(OCH2CH2)3OH
CAS Number:
Molecular Weight:
336.53
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Assay
95%
refractive index
n20/D 1.476
density
0.995 g/mL at 25 °C
storage temp.
−20°C
SMILES string
OCCOCCOCCOCCCCCCCCCCCS
InChI
1S/C17H36O4S/c18-10-12-20-14-16-21-15-13-19-11-8-6-4-2-1-3-5-7-9-17-22/h18,22H,1-17H2
InChI key
FASSFROSROBIBE-UHFFFAOYSA-N
General description
Triethylene glycol mono-11-mercaptoundecyl ether is an alkanethiol that forms a self-assembled monolayer (SAM) on a variety of surfaces.
Application
Triethylene glycol mono-11-mercaptoundecyl ether can be used in the formation of low cost micro-patterns for biomedical and microelectronics based applications. It can also be used as a SAM for surface immobilization of various molecular species on gold electrodes for biological applications.
Used in an efficient synthesis of ω-functionalized asymmetric disulfides via the sulfenyl bromide.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
>230.0 °F
Flash Point(C)
> 110 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Janusz Kowalczyk et al.
Langmuir : the ACS journal of surfaces and colloids, 23(5), 2318-2321 (2007-01-26)
Various types of asymmetric disulfides can be synthesized under mild conditions and in excellent yields by a method involving dialkoxylthiophosphoranesulfenyl halide precursors. This straightforward, rapid procedure is used to prepare a series of disulfides bearing neutral, acidic, and basic terminal
Optically transparent, amphiphilic networks based on blends of perfluoropolyethers and poly (ethylene glycol).
Hu Z, et al.
Journal of the American Chemical Society, 130(43), 14244-14252 (2008)
Protein resistance of surfaces modified with oligo (ethylene glycol) aryl diazonium derivatives.
Fairman C, et al.
ChemPhysChem, 14(10), 2183-2189 (2013)
Bio-Inspired Silicification on Patterned Surfaces Generated by Microcontact Printing and Layer-by-Layer Self-Assembly.
Yang SH and Choi IS
Chemistry - An Asian Journal, 4(3), 382-385 (2009)
Highly sensitive detection of pathogen Escherichia coli O157: H7 by electrochemical impedance spectroscopy.
Dos S, et al.
Biosensors And Bioelectronics, 45(3), 174-180 (2013)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![2-{2-[2-(2-Mercaptoethoxy)ethoxy]ethoxy}ethanol 97%](/deepweb/assets/sigmaaldrich/product/structures/130/969/7d2ec2b4-e0f1-4836-aeb2-139994173612/640/7d2ec2b4-e0f1-4836-aeb2-139994173612.png)