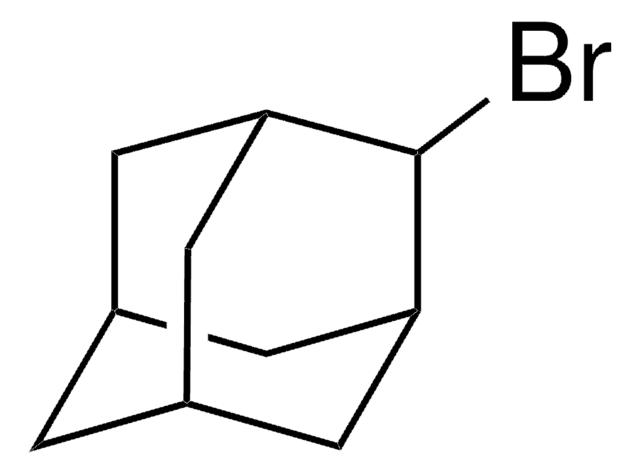
377198
1-Iodoadamantane
98%
Synonym(s):
1-Adamantyl iodide, 1-Iodotricyclo[3.3.1.13,7]decane, Adamantyl iodide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C10H15I
CAS Number:
Molecular Weight:
262.13
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
solid
mp
75-76 °C (lit.)
SMILES string
IC12C[C@H]3C[C@H](C[C@H](C3)C1)C2
InChI
1S/C10H15I/c11-10-4-7-1-8(5-10)3-9(2-7)6-10/h7-9H,1-6H2/t7-,8+,9-,10-
InChI key
PXVOATXCSSPUEM-CHIWXEEVSA-N
General description
1-Iodoadamantane is a haloadamantane. Voltammetric reduction of 1-iodoadamantane at a silver cathode in tetrahydrofuran (THF) and acetonitrile (ACN) is reported to involve a single electron forming a mixture of monomeric and dimeric products. The photoinduced reaction of 1-iodoadamantane in DMSO is reported to afford substitution products on C3, C6, and C8, 1-adamantanol, 1-adamantyl 2-naphthyl ether, and adamantine. The photostimulated reaction of the phthalimide anion with 1-iodoadamantane is reported to yield 3-(1-adamantyl) phthalimide and 4-(1-adamantyl) phthalimide, along with the reduction product adamantane. 1-Iodoadamantane is reported to undergoe photostimulated reaction with the enolate anion of acetone, acetophenone and propiophenone to give admantane and the substitution products.
Application
1-Iodoadamantane is suitable reagent used to evaluate the rate constants for the reduction of haloadamantanes by SmI2 in presence of hexamethylphosphoramide (HMPA) and H2O by GC/MS-analyzed method. It may be used as iodine atom donor for probing the intermediacy of radical to investigate the chemistry of highly reactive, strained systems such as propellane. It may be used as starting reagent in the synthesis of N-(1-adamantyl)acetamide via nucleophilic substitution. It may be employed in the free-radical carbonylation reactions with alkenes.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Christopher A Paddon et al.
Ultrasonics sonochemistry, 14(5), 502-508 (2007-01-17)
The combination of ultrasound and electrochemistry -sonoelectrochemistry can produce a variety of effects within an electrochemical system including enhanced mass transport, in situ cleaning of an electrode surface, diminution of the diffusion layer, and possible induction of new reactions by
Robert Vícha et al.
Acta crystallographica. Section E, Structure reports online, 65(Pt 6), o1307-o1307 (2009-01-01)
In the title salt, C(12)H(20)NO(+)·CH(3)SO(3) (-), the [1-(1-adamantyl-amino)ethyl-idene]oxonium cations and methane-sulfonate anions are linked into chains along the a axis via O-H⋯O and N-H⋯O hydrogen bonds. All non-H atoms of the acetamido group are essentially planar, with a maximum deviation
Photostimulated reaction of 1-iodoadamantane with carbanionic nucleophiles in DMSO by the SRN1 mechanism.
Borosky GL, et al.
The Journal of Organic Chemistry, 55(12), 3705-3707 (1990)
Rate study of haloadamantane reduction by samarium diiodide.
Lin T-Y, et al.
J. Chin. Chem. Soc., 49(6), 969-973 (2002)
Juan E Argüello et al.
The Journal of organic chemistry, 68(6), 2362-2368 (2003-03-15)
The fluorescent excited state of the 2-naphthoxide ion (1) is quenched by aliphatic and aromatic halides according to an electron-transfer mechanism, with generation of the corresponding alkyl and aryl radicals by a concerted or consecutive C-X bond fragmentation reaction. Whereas
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service