560529
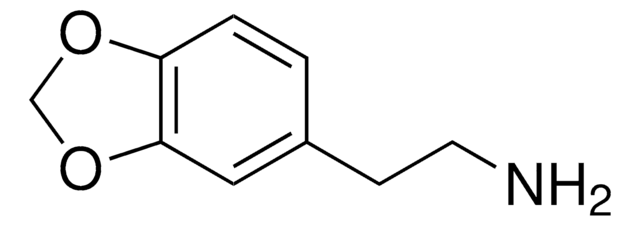
3,4-Methylenedioxyphenethylamine hydrochloride
98%
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H11NO2 · HCl
CAS Number:
Molecular Weight:
201.65
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
mp
216-218 °C (lit.)
SMILES string
Cl.NCCc1ccc2OCOc2c1
InChI
1S/C9H11NO2.ClH/c10-4-3-7-1-2-8-9(5-7)12-6-11-8;/h1-2,5H,3-4,6,10H2;1H
InChI key
NDYXFQODWGEGNU-UHFFFAOYSA-N
General description
3,4-Methylenedioxyphenethylamine hydrochloride can be synthesized by reacting aluminum chloride, LiAlH4 and 3,4-methylenedioxyphenylacetonitrile.
Application
3,4-Methylenedioxyphenethylamine hydrochloride may be used to synthesize N-(3,4-methylenedioxyphenethyl)-2-(3-bromo-4-methoxyphenyl)acetamide.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Jan G Bruhn et al.
Journal of psychoactive drugs, 40(2), 219-222 (2008-08-30)
Human interest in psychoactive phenethylamines is known from the use of mescaline-containing cacti and designer drugs such as Ecstasy. From the alkaloid composition of cacti we hypothesized that substances resembling Ecstasy might occur naturally. In this article we show that
An aryne route to laureline, and related topics.
Gibson MS, et al.
J. Chem. Soc. Sect. C, 16, 2234-2238 (1970)
D J De Silva et al.
Neuroscience, 134(4), 1363-1375 (2005-08-02)
Substituted amphetamines such as p-chloroamphetamine and the abused drug methylenedioxymethamphetamine cause selective destruction of serotonin axons in rats, by unknown mechanisms. Since some serotonin neurones also express neuronal nitric oxide synthase, which has been implicated in neurotoxicity, the present study
Kjell A Mortier et al.
Rapid communications in mass spectrometry : RCM, 16(9), 865-870 (2002-04-12)
Paramethoxyamphetamine (PMA) is an amphetamine-like designer drug that has emerged recently on the European illicit drug market. This drug has a wicked reputation, as a number of lethal intoxications have occurred. A method using high-performance liquid chromatography coupled to ion
Jon E Sprague et al.
The Journal of pharmacology and experimental therapeutics, 305(1), 159-166 (2003-03-22)
An acute and potentially life-threatening complication associated with the recreational use of the 3,4-methylenedioxymethamphetamine (MDMA, Ecstasy) is hyperthermia. In the present study, Sprague-Dawley rats treated with MDMA (40 mg/kg s.c.) responded with a significant increase (maximal at 1 h) in
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service