72420
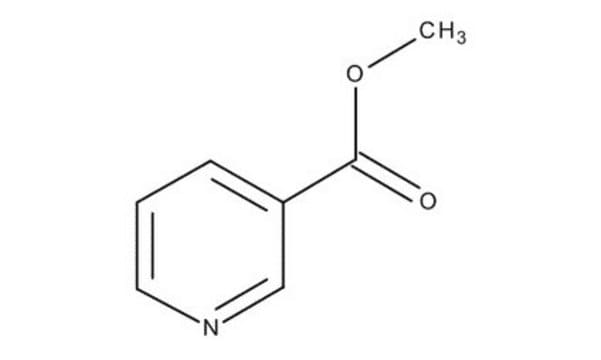
Methyl nicotinate
puriss., ≥99.0% (GC)
Synonym(s):
Nicotinic acid methyl ester
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C7H7NO2
CAS Number:
Molecular Weight:
137.14
Beilstein:
113951
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
biological source
synthetic
Quality Level
grade
puriss.
Assay
≥99.0% (GC)
form
powder or crystals (possibly with chunks)
color
white to faint yellow
bp
204 °C (lit.)
mp
39-42 °C
42-44 °C (lit.)
solubility
H2O: 0.1 g/mL, clear
SMILES string
COC(=O)c1cccnc1
InChI
1S/C7H7NO2/c1-10-7(9)6-3-2-4-8-5-6/h2-5H,1H3
InChI key
YNBADRVTZLEFNH-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Methyl nicotinate (or nicotinic acid methyl ester) is used as a rubefacient for the relief of pains in muscles, tendons, and joints. It is also used in food as a flavoring agent.
Application
Methyl nicotinate can be used as a precursor:
- In the asymmetric synthesis of 1-azasugars (glycosidase inhibitors) for biomedical applications.
- In the total synthesis of ±-sesbanine.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Topical antirheumatic agents as hydroxyl radical scavengers
Billany MR, et al.
International Journal of Pharmaceutics, 124(2), 279-283 (1995)
A facile synthesis of ? -sesbanine via γ-addition of ketene silyl acetal with quaternized methyl nicotinate
Wada M, et al.
Tetrahedron Letters, 26(27), 3267-3270 (1985)
Selective fowler reductions: asymmetric total syntheses of isofagomine and other 1-azasugars from methyl nicotinate
Zhao G, et al.
Organic Letters, 3(2), 201-203 (2001)
Stability of methylnicotinate in aqueous solution as utilized in the'niacin patch test'
Ross BM and Katzman M
BMC Research Notes, 1(1), 89-89 (2008)
Identification and quantification of methyl nicotinate in rice (Oryza sativa L.) by gas chromatography-mass spectrometry
RB Muralidhara, et al.
Food Chemistry, 105(2), 736-741 (2007)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service