All Photos(1)
About This Item
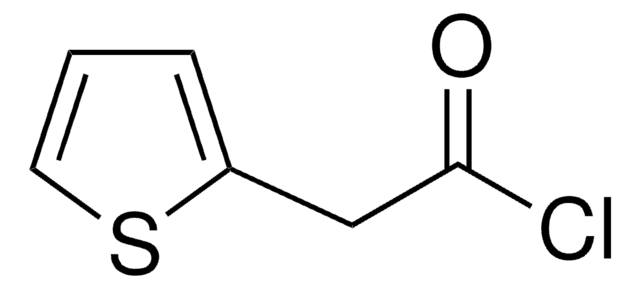
Linear Formula:
(C2H5)2NCSCl
CAS Number:
Molecular Weight:
151.66
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
95%
form
solid
bp
113 °C/10 mmHg (lit.)
mp
45-50 °C (lit.)
SMILES string
CCN(CC)C(Cl)=S
InChI
1S/C5H10ClNS/c1-3-7(4-2)5(6)8/h3-4H2,1-2H3
InChI key
HUUSTUALCPTCGJ-UHFFFAOYSA-N
Related Categories
General description
Diethylthiocarbamoyl chloride is a thiocarbamoyl derivative. It has been reported to participate in the thionation of amides as thionating reagent.
Application
Diethylthiocarbamoyl chloride (N,N′-Diethylthiocarbamoyl chloride) may be used in the following syntheses:
- aryl isothiocyanates
- 5-substituted 4-methyl-2-thiazolyl diethyldithiocarbamates
- novel type of vasorelaxant hybrid compounds
Diethylthiocarbamoyl chloride may be used in the synthesis of 2,6-dimethyl-3,5-dicarbomethoxy-4-(2-difluoromethoxy- 5-isothiocyanatophenyl)-1,4-dihydropyridine.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Derivatives of thiazolethiols.
D'Amico JJ, et al.
Journal of the American Chemical Society, 79(19), 5270-5276 (1957)
The Reaction of Arylamines with Diethylthiocarbamoyl Chloride. A New Synthesis of Aryl Isothiocyanates.
Sayigh AAR, et al.
The Journal of Organic Chemistry, 30(7), 2465-2466 (1965)
Sulfated tungstate: An efficient catalyst for synthesis of thioamides via Kindler reaction.
Pathare SP, et al.
Applied Catalysis A: General, 425, 125-129 (2012)
L M Yagupolskii et al.
Journal of medicinal chemistry, 42(25), 5266-5271 (1999-12-22)
The synthesis and pharmacological properties of a novel type of vasorelaxant hybrid compounds are described. The investigated compounds originate from fluorinated 4-aryl-1,4-dihydropyridines, which are known calcium channel blockers, and/or from fluorinated analogues of pinacidil, which is an opener of ATP-sensitive
K R Frazier et al.
Journal of applied microbiology, 126(1), 79-86 (2018-08-31)
Disulfiram (Antabuse™) and its metabolites formed in vivo were evaluated as antibacterial agents against thirty species of Gram-positive and Gram-negative bacteria. The synergistic potential of disulfiram (DSF) and metabolite diethyldithiocarbamate (DDTC) with approved antibiotics were also compared by isobologram (checkerboard) analysis.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service