214027
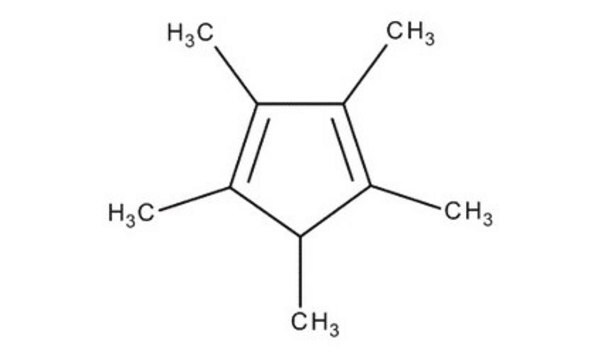
1,2,3,4,5-Pentamethylcyclopentadiene
95%
Synonym(s):
1,2,3,4,5-Pentamethyl-1,3-cyclopentadiene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C10H16
CAS Number:
Molecular Weight:
136.23
Beilstein:
1849832
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
refractive index
n20/D 1.474 (lit.)
bp
58 °C/13 mmHg (lit.)
density
0.87 g/mL at 25 °C (lit.)
SMILES string
CC1C(C)=C(C)C(C)=C1C
InChI
1S/C10H16/c1-6-7(2)9(4)10(5)8(6)3/h6H,1-5H3
InChI key
WQIQNKQYEUMPBM-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Mechanism of the Diels-Alder reaction of paramagneticendohedral metallofullerene and 1,2,3,4,5-pentamethylcyclopentadiene has been studied. Three-step large-scale preparation of 1,2,3,4,5-pentamethylcyclopentadiene has been reported. Reaction of 1,2,3,4,5-pentamethylcyclopentadiene (HCp*) with actinide ions in gas phase has been investigated by laser ablation mass spectrometry. It undergoes radical cation catalyzed cycloaddition with electron rich allenes to form Diels-Alder product.
Application
1,2,3,4,5-pentamethylcyclopentadiene was used as:
- Growth modifier chemical, during metal organic chemical vapour deposition of iron from iron pentacarbonyl.
- Ligand in "one-pot" iridium-catalyzed transformation of alcohols to amides via the intermediacy of oximes.
- Raw material for the synthesis of [Cp*Rh(bpy)H2O]2+ (Cp* = pentamethylcyclopentadienyl, bpy = 2,2′-bipyridyl), an electron mediator in the regeneration process of NADH.
Signal Word
Warning
Hazard Statements
Hazard Classifications
Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
111.2 °F - closed cup
Flash Point(C)
44 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Journal of Organometallic Chemistry, 472, 359-359 (1994)
Nathan A Owston et al.
Organic letters, 9(1), 73-75 (2006-12-29)
[reaction: see text] The iridium catalyst [Ir(Cp*)Cl2]2 is effective for the rearrangement of oximes to furnish amides. The reaction has been combined with catalytic transfer hydrogenation between an alcohol and alkene to allow the conversion of alcohols into amides in
The Aminium Salt and Photoinduced Electron Transfer Initiated Diels-Alder Cycloaddition of Electron-rich Allenes: Evidence for a Stepwise Mechanism and the Importance of Steric and Electronic Effects for the Reactivity of Distonic Radical Cation Intermediates.
Schmittel M, et al.
Chemistry (Weinheim An Der Bergstrasse, Germany), 2(8), 1031-1040 (1996)
An improved synthesis of 1,2,3,4,5-pentamethylcyclopentadiene.
Kohl FX and Jutzi P.
Journal of Organometallic Chemistry, 243(1), 119-121 (1983)
Synth. React. Inorg. Met.-Org. Chem. , 24, 395-395 (1994)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service