All Photos(1)
About This Item
Linear Formula:
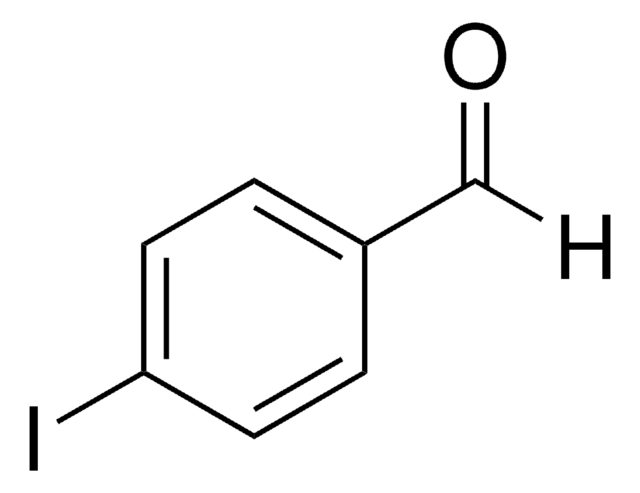
IC6H4CHO
CAS Number:
Molecular Weight:
232.02
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
97%
mp
36-39 °C (lit.)
storage temp.
2-8°C
SMILES string
Ic1ccccc1C=O
InChI
1S/C7H5IO/c8-7-4-2-1-3-6(7)5-9/h1-5H
InChI key
WWKKTHALZAYYAI-UHFFFAOYSA-N
Related Categories
General description
2-Iodobenzaldehyde (o-iodobenzaldehyde) is a 2-halobenzaldehyde derivative. Its crystals belong to the orthorhombic crystal system and P212121 space group.
Application
2-Iodobenzaldehyde may be used as a reactant in the synthesis of the following heterocycles:
- 2,3-diaryl-1-indenones
- indolo[1,2-a]quinazolines
- Baylis-Hillman (BH) adducts
- 5-phenylindazolo[3,2-b]quinazolin-7(5H)-one
- 4-(3-iodophenyl)-2,2:6,2-terpyridine
- fluoren-9-one
- 2-formyl-3′-methoxybiphenyl
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
2-Iodobenzaldehyde.
Betz R and Klufers P.
Acta Crystallographica Section E, Structure Reports Online, 63(12), o4879-o4879 (2007)
Ring-Closing Olefin Metathesis of 2, 2'-Divinylbiphenyls: A Novel and General Approach to Phenanthrenes.
Iuliano A, et al.
Organic Letters, 6(21), 3711-3714 (2004)
Copper (I)-Catalyzed Synthesis of 5-Arylindazolo [3, 2-b] quinazolin-7 (5 H)-one via Ullmann-Type Reaction
Chen DS, et al.
The Journal of Organic Chemistry, 78(11), 5700-5704 (2013)
Combined catalysis: Pd-catalyzed two-step one-pot protocol for 2, 3-diaryl-1-indenones involving domino synthesis of diarylacetylenes and Heck-Larock annulations.
Rao MLN and Dhanorkar RJ.
Tetrahedron, 70(43), 8067-8078 (2014)
Self-Assembly of Shape-Persistent Hexagonal Macrocycles with Trimeric Bis (terpyridine)-FeII Connectivity.
Li S, et al.
European Journal of Organic Chemistry, 2008(19), 3328-3334 (2008)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service