424714
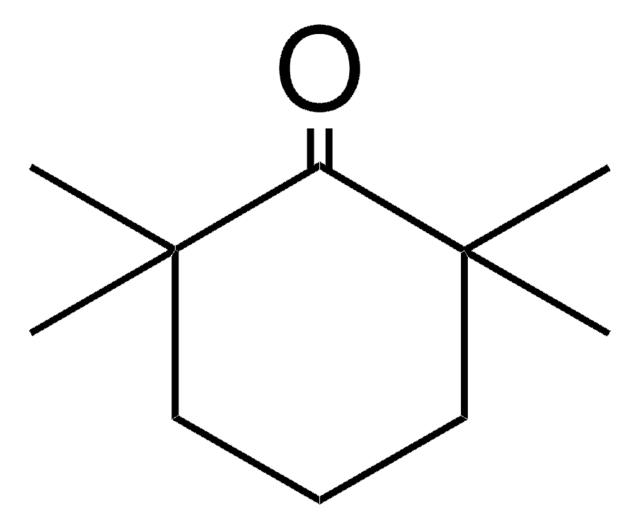
2,6-Diphenylcyclohexanone, mixture of cis and trans
97%
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
(C6H5)2C6H8(=O)
CAS Number:
Molecular Weight:
250.33
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
assay:
97%
Recommended Products
assay
97%
mp
119-121 °C (lit.)
functional group
ketone
phenyl
SMILES string
O=C1C(CCCC1c2ccccc2)c3ccccc3
InChI
1S/C18H18O/c19-18-16(14-8-3-1-4-9-14)12-7-13-17(18)15-10-5-2-6-11-15/h1-6,8-11,16-17H,7,12-13H2
InChI key
JHMUMWBKYPMOLI-UHFFFAOYSA-N
General description
2,6-Diphenylcyclohexanone is a cyclohexanone with two phenyl substituents at two α-positions. 2,6-Diphenylcyclohexanone is the starting material employed in the synthesis of cis- and trans-2-(p-carboxybenzyl)-2,6-diphenyl-6-vinylcyclohexanone. cis -2,6-Diphenylcyclohexanone is photochemically active and it undergoes photodecarbonylation reactions.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Engineering reactions in crystals: suppression of photodecarbonylation by intramolecular β-phenyl quenching.
Ng D, et al.
Tetrahedron Letters, 42(52), 9113-9116 (2001)
Horspool WM and Lenci F.
CRC Handbook of Organic Photochemistry and Photobiology, 1 -2, 48-48 (2003)
Platz MS, et al.
Reviews of Reactive Intermediate Chemistry, 310-312 (2007)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service