All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C3H4N2O
CAS Number:
Molecular Weight:
84.08
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
95%
form
liquid
refractive index
n20/D 1.511 (lit.)
bp
226-228 °C (lit.)
density
1.138 g/mL at 25 °C (lit.)
SMILES string
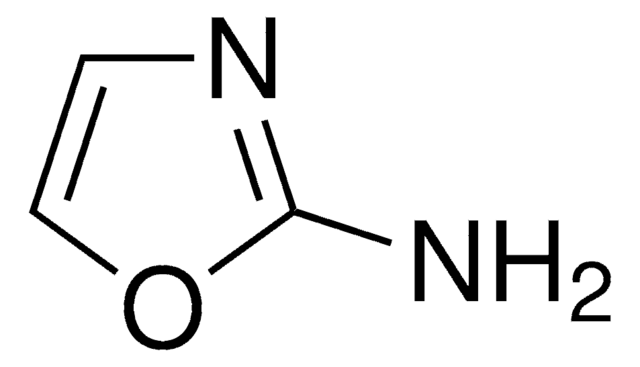
Nc1ccon1
InChI
1S/C3H4N2O/c4-3-1-2-6-5-3/h1-2H,(H2,4,5)
InChI key
RHFWLPWDOYJEAL-UHFFFAOYSA-N
General description
3-Aminoisoxazole (isoxazol-3-amine) is a 3-substituted isoxazole derivative. It is a structural isomer of 5-aminoisoxazole.
Application
3-Aminoisoxazole (isoxazol-3-amine) may be used in the following studies:
- As a reagent in the synthesis of N-(4-(N-isoxazol-3-ylsulfamoyl)phenyl)acetamide.
- As a starting material in the synthesis of N-(isoxazol-3-yl)-N′-(carbomethoxy)thiourea.
- As a starting material in the synthesis of (Z)-2-(5-amino-1,2,4-thiadiazol-3-yl)-2-methoxy-iminoacetic acid, a side-chain of the fourth generation of cephem antibiotics.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
235.4 °F
flash_point_c
113 °C
ppe
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Kuniaki Tatsuta
Proceedings of the Japan Academy. Series B, Physical and biological sciences, 84(4), 87-106 (2008-10-23)
The first total synthesis and development of a variety of bioactive natural products have been accomplished by using carbohydrates as a chiral source. In addition, practically useful intermediates have been created, analogs of natural products have been prepared, their structure-activity
Tomasz Glinka et al.
Bioorganic & medicinal chemistry, 11(4), 591-600 (2003-01-23)
SAR studies in a series of related 3-(heteroarylthio)cephems determined that a relatively high chemical reactivity of the beta-lactam ring, modulated by electronic effects of substituents at C-3 and C-7, is necessary to achieve high in vitro activity against methicillin-resistant Staphylococcus
Synthesis of 3-and 5-amino-5-(3)-(pyrrol-2-yl) isoxazoles.
Lyubov'N S, et al.
Tetrahedron, 61(20), 4841-4849 (2005)
Martin J. Walsh et al.
Probe Reports from the NIH Molecular Libraries Program, 2011 Oct 18 (Updated 2013 Feb 25) (2013-09-13)
The protist
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service