U5625
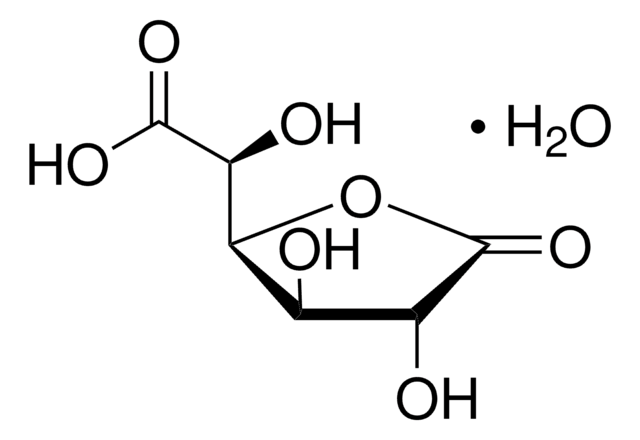
Uridine 5′-diphosphoglucuronic acid ammonium salt
98-100%
Synonym(s):
UDP-GlcA, UDPGA, Uridine-diphosphate-glucuronic acid ammonium salt, Uridine[5′]diphospho[1]-α-D-glucopyranosuronic acid ammonium salt
Select a Size
$420.00
In StockDetails
Select a Size
About This Item
$420.00
In StockDetails
Recommended Products
biological source
Saccharomyces cerevisiae
enzyme from bovine liver (catalase)
enzyme from rabbit muscle (LDH)
assay
98-100%
form
powder
storage temp.
−20°C
SMILES string
N.O[C@@H]1[C@@H](O)[C@H](O[C@@H]([C@H]1O)C(O)=O)OP(O)(=O)OP(O)(=O)OC[C@H]2O[C@H]([C@H](O)[C@@H]2O)N3C=CC(=O)NC3=O
InChI
1S/C15H22N2O18P2.H3N/c18-5-1-2-17(15(26)16-5)12-9(22)6(19)4(32-12)3-31-36(27,28)35-37(29,30)34-14-10(23)7(20)8(21)11(33-14)13(24)25;/h1-2,4,6-12,14,19-23H,3H2,(H,24,25)(H,27,28)(H,29,30)(H,16,18,26);1H3/t4-,6-,7+,8+,9-,10-,11+,12-,14-;/m1./s1
InChI key
WMWKTCPGFOEPBD-YGIWDPDDSA-N
1 of 4
This Item | U5252 | U6751 | U4375 |
|---|---|---|---|
| Quality Level 200 | Quality Level 200 | Quality Level 300 | Quality Level 200 |
| assay 98-100% | assay ≥97% | assay 98-100% | assay ≥98% |
| form powder | form powder | form powder | form powder |
| storage temp. −20°C | storage temp. −20°C | storage temp. −20°C | storage temp. −20°C |
General description
Application
Biochem/physiol Actions
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Explore tools for glycosyltransferase synthesis and modification of glycans, such as glycosyltransferases and nucleotide sugar donors.
LC-MS/MS method quantifies similar polar nucleotide activated sugars using Supel™ Carbon LC column for simultaneous analysis.
Enzymatic glycosyltransferase specificity challenges the one enzyme-one linkage concept.
Active Filters
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service