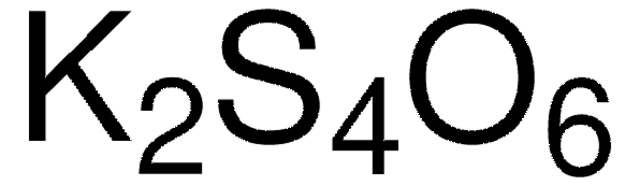About This Item
Recommended Products
Quality Level
assay
≥98% (titration)
solubility
H2O: 200 + 4mL H2O mg, clear to hazy, colorless to faintly yellow
density
2.1 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
O.O.[Na+].[Na+].[O-]S(=O)(=O)SSS([O-])(=O)=O
InChI
1S/2Na.H2O6S4.2H2O/c;;1-9(2,3)7-8-10(4,5)6;;/h;;(H,1,2,3)(H,4,5,6);2*1H2/q2*+1;;;/p-2
InChI key
HAEPBEMBOAIUPN-UHFFFAOYSA-L
Looking for similar products? Visit Product Comparison Guide
Application
- Gaussia princeps luciferase: a bioluminescent substrate for oxidative protein folding.: Sodium tetrathionate dihydrate is used to study its impact on the oxidative folding of proteins, utilizing Gaussia princeps luciferase as a bioluminescent substrate. This study advances understanding of protein chemistry and bioluminescence applications in biochemical assays (Yu T et al., 2018).
- A spectroscopic investigation into the reaction of sodium tetrathionate with cysteine.: The research utilizes spectroscopic methods to study the reaction between sodium tetrathionate dihydrate and cysteine, providing insights into the chemical interactions and potential applications in biochemistry and molecular biology (Church JS et al., 2008).
https://doi.org/10.2174/092986612800190973
https://doi.org/10.1080/10826068.2011.544230
https://doi.org/10.11150/kansenshogakuzasshi.83.380
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service