T89605
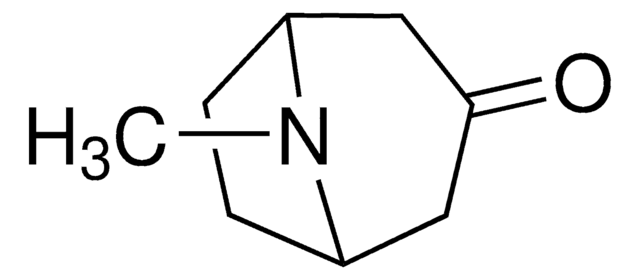
Tropinone
99%
Synonym(s):
8-Methyl-8-azabicyclo[3.2.1]octan-3-one
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C8H13NO
CAS Number:
Molecular Weight:
139.19
Beilstein/REAXYS Number:
2329
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
99%
bp
113 °C/25 mmHg (lit.)
mp
40-44 °C (lit.)
storage temp.
2-8°C
SMILES string
CN1[C@@H]2CC[C@H]1CC(=O)C2
InChI
1S/C8H13NO/c1-9-6-2-3-7(9)5-8(10)4-6/h6-7H,2-5H2,1H3/t6-,7+
InChI key
QQXLDOJGLXJCSE-KNVOCYPGSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral
Storage Class
11 - Combustible Solids
wgk_germany
WGK 1
flash_point_f
194.0 °F - closed cup
flash_point_c
90 °C - closed cup
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Capturing enzyme structure prior to reaction initiation: tropinone reductase-II-substrate complexes.
Atsuko Yamashita et al.
Biochemistry, 42(19), 5566-5573 (2003-05-14)
To understand the catalytic mechanism of an enzyme, it is crucial to determine the crystallographic structures corresponding to the individual reaction steps. Here, we report two crystal structures of enzyme-substrate complexes prior to reaction initiation: tropinone reductase-II (TR-II)-NADPH and TR-II-NADPH-tropinone
Enantioselective synthesis of unnatural (S)-(+)-cocaine.
J C Lee et al.
The Journal of organic chemistry, 65(15), 4773-4775 (2000-08-26)
Franklin A Davis et al.
Organic letters, 11(7), 1647-1650 (2009-03-13)
Sulfinimine-derived, enantiopure N-sulfinyl beta-amino ketone ketals on hydrolysis give dehydropyrrolidine ketones that on treatment with (Boc)(2)O/DMAP afford substituted tropinones in good yield.
E Leete
Planta medica, 56(4), 339-352 (1990-08-01)
Recent work on the biosynthesis of the tropane moiety of cocaine, hyoscamine, scopolamine, and related alkaloids is reviewed. Revision of the generally accepted biosynthetic pathway to these alkaloids is now proposed in the light of new discoveries. New information on
A Portsteffen et al.
Phytochemistry, 37(2), 391-400 (1994-09-01)
In tropane-alkaloid producing plants and root cultures, the reduction of tropinone is a branch-point in secondary metabolism. Two different reductases stereospecifically form the isomeric alcohols tropine (tropan-3 alpha-ol) and pseudotropine (tropan-3 beta-ol). We describe here the purification and characterization of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service