All Photos(1)
About This Item
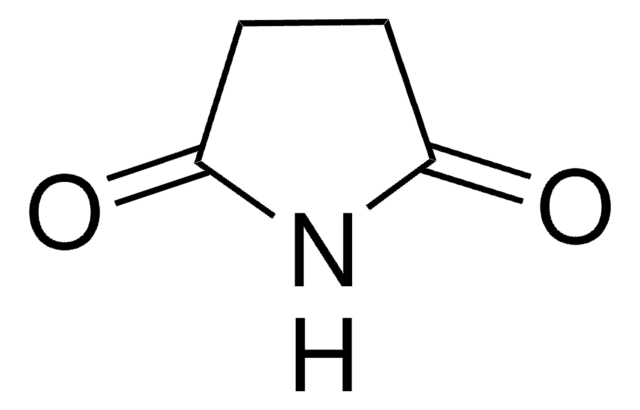
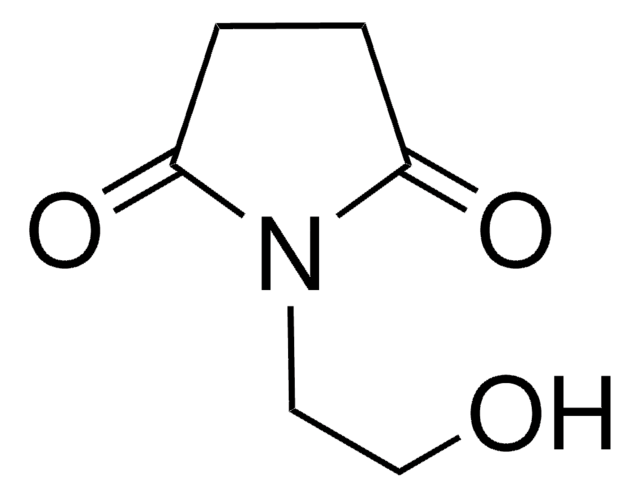
Empirical Formula (Hill Notation):
C4H5NO2
CAS Number:
Molecular Weight:
99.09
Beilstein/REAXYS Number:
108440
EC Number:
MDL number:
UNSPSC Code:
12352103
Recommended Products
assay
98%
bp
285-290 °C (lit.)
mp
123-125 °C (lit.)
SMILES string
O=C1CCC(=O)N1
InChI
1S/C4H5NO2/c6-3-1-2-4(7)5-3/h1-2H2,(H,5,6,7)
InChI key
KZNICNPSHKQLFF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
replaced by
Product No.
Description
Pricing
Storage Class
11 - Combustible Solids
wgk_germany
WGK 1
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Zhiwei Lin et al.
Physical chemistry chemical physics : PCCP, 14(30), 10445-10454 (2012-05-05)
Dual-frequency relaxation-assisted two-dimensional infrared (RA 2DIR) spectroscopy was used to investigate energy transport in polyethylene glycol (PEG) oligomers of different length, having 0, 4, 8, and 12 repeating units and end-labeled with azido and succinimide ester moieties (azPEGn). The energy
Yutaka Sadakane et al.
Journal of chromatography. B, Analytical technologies in the biomedical and life sciences, 879(29), 3240-3246 (2011-04-08)
The major soluble eye lens protein, αA-crystallin, has a very long half-life. Thus, many post-translational modifications, including stereoinversion, have been found in its constituent amino acids. We determine the rates of β-linkage isomerization, which is the main reaction through the
Yann Desfougères et al.
Biomacromolecules, 12(1), 156-166 (2010-12-21)
Protein chemical degradations occur naturally into living cells as soon as proteins have been synthesized. Among these modifications, deamidation of asparagine or glutamine residues has been extensively studied, whereas the intermediate state, a succinimide derivative, was poorly investigated because of
Ohgi Takahashi et al.
Chemistry & biodiversity, 7(6), 1630-1633 (2010-06-22)
The rapid racemization of aspartic acid (Asp) residues in peptides and proteins is due mainly to the succinimide intermediate. However, there should be another mechanism for Asp racemization without intermediacy of the succinimide. The direct H-atom abstraction from the C(alpha)-atom
Srinivasa Reddy Mothe et al.
The Journal of organic chemistry, 76(8), 2521-2531 (2011-03-15)
A one-pot, two-step method to prepare 3-halohydrofurans efficiently by TfOH-catalyzed hydroxylation/halocyclization of cyclopropyl methanols with H(2)O and N-halosuccinimide (NXS, X=1, Br, Cl) or Selectfluor is described. The reactions proceed rapidly under mild and operationally straightforward conditions with a catalyst loading
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service