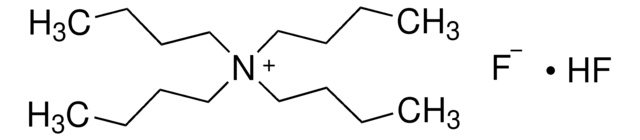
86856
Tetrabutylammonium hydrogen difluoride solution
technical, ~50% in methylene chloride (T)
Synonym(s):
TBABF, Tetrabutylammonium bifluoride, Tetrabutylammonium difluoride
About This Item
Recommended Products
grade
technical
form
liquid
concentration
~50% in methylene chloride (T)
impurities
~5% tetrabutylammonium chloride
refractive index
n20/D 1.442
storage temp.
2-8°C
SMILES string
[F-].F[H].CCCC[N+](CCCC)(CCCC)CCCC
InChI
1S/C16H36N.2FH/c1-5-9-13-17(14-10-6-2,15-11-7-3)16-12-8-4;;/h5-16H2,1-4H3;2*1H/q+1;;/p-1
InChI key
ZHBDKVWQJKYIFF-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
Application
- Used as an etchant for silica films
Reactant for:
- Nucleophilic fluorination
- Ring-opening fluorination reaction
- Substitution reactions
- For the conversion of aromatic boronic acids and esters into corresponding tetrabutylammonium trifluoroborate salts. These salts are utilized as surrogates of boronic acids in Suzuki-Miyaura cross-coupling reactions.
- In the nucleophilic fluorination of organic halides, tosylates, and triflates.
- For the regioselective synthesis of fluorohydrins by ring-opening of terminal epoxides.
signalword
Danger
hcodes
Hazard Classifications
Carc. 2 - Eye Dam. 1 - Skin Corr. 1B - STOT SE 3
target_organs
Central nervous system
Storage Class
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service