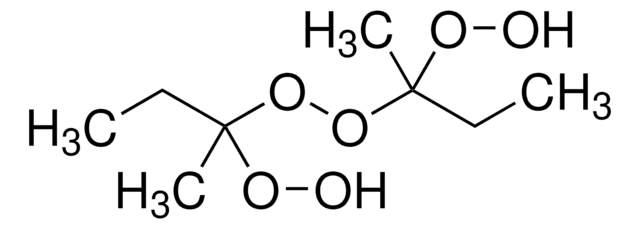
743003
2-Butanone peroxide
technical
Synonym(s):
Ethyl methyl ketone peroxide, Methyl ethyl ketone peroxide
About This Item
Recommended Products
grade
technical
form
liquid
contains
2,2,4-trimethyl-1,3-pentanediol-diisobutyrate as stabilizer
reaction suitability
reagent type: oxidant
refractive index
n20/D 1.432
density
1.053 g/mL at 20 °C (lit.)
functional group
hydroperoxide
peroxide
storage temp.
2-8°C
SMILES string
CCC(C)(OO)OOC(C)(CC)OO
InChI
1S/C8H18O6/c1-5-7(3,11-9)13-14-8(4,6-2)12-10/h9-10H,5-6H2,1-4H3
InChI key
WFUGQJXVXHBTEM-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- Studies of the adiabatic runaway reaction of Me Et Ketone peroxide
- Quantitation of metal ions in archaeological glass via abrasive stripping square-wave voltammetry
- Imaging of hydrogen peroxide and hydrogen peroxide-scavenging substances by photon emission
- Synthesis of oligonucleotides via phosphoramidite method
- Oxidation of nucleoside phosphites into phosphates
- Comparing the relative effectiveness of human plasma glutathione peroxidase as a catalyst for reduction of hydroperoxidases
Analyte for direct electrochemical catalystis of immobilized Hb in an ethanol-water mixture
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Flam. Liq. 3 - Org. Perox. D - Skin Corr. 1B
Storage Class
5.2 - Organic peroxides and self-reacting hazardous materials
wgk_germany
WGK 1
flash_point_f
132.8 °F - closed cup
flash_point_c
56 °C - closed cup
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service