73753
1,7-Diaza-12-crown-4
≥97.0%
Synonym(s):
1,7-Dioxa-4,10-diazacyclododecane
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C8H18N2O2
CAS Number:
Molecular Weight:
174.24
Beilstein/REAXYS Number:
606115
EC Number:
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
assay
≥97.0%
form
crystals
mp
80-84 °C
SMILES string
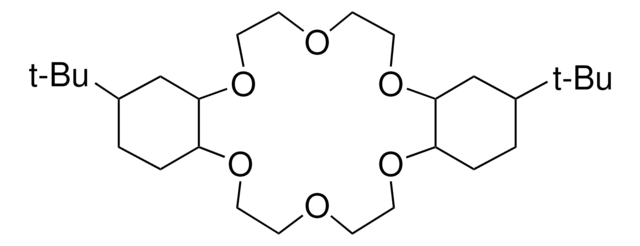
C1COCCNCCOCCN1
InChI
1S/C8H18N2O2/c1-5-11-7-3-10-4-8-12-6-2-9-1/h9-10H,1-8H2
InChI key
PWJHXHMUGFXPSN-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
1,7-Diaza-12-crown-4 can be used to prepare:
- A ligand named N,N′-bis[(6-carboxy-2-pyridyl)methyl]-1,7-diaza-12-crown-4, which is used to synthesize lanthanide metal complexes applicable as MRI contrast agents.
- A novel chiral receptor that can be used in the separation of enantiomers using capillary electrophoresis.
- A fluorescent anthracene based diazacrown ether, which can be used as a photoinduced electron transfer (PET) fluorescent sensor for guest cations.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Lanthanide complexes based on a 1, 7-diaza-12-crown-4 platform containing picolinate pendants: a new structural entry for the design of magnetic resonance imaging contrast agents
Mato-Iglesias M, et al.
Inorganic Chemistry, 47(17), 7840-7851 (2008)
Complexation and fluorescence behavior of diazacrown ether carrying two anthryl pendants
Kubo K, et al.
Talanta, 49(2), 339-344 (1999)
Synthesis of a new chiral receptor containing 1, 7-diaza-12-crown-4 and its application in chiral separation
Wang C, et al.
Synthetic Communications, 33(19), 3381-3386 (2003)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service