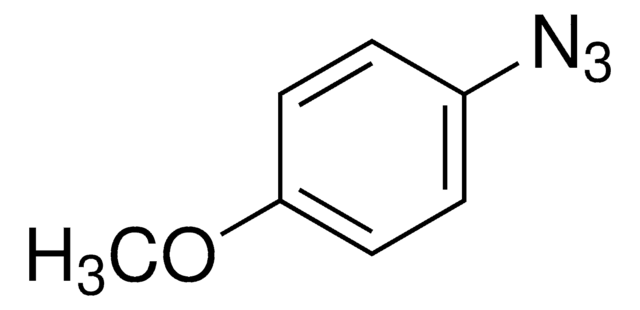
727490
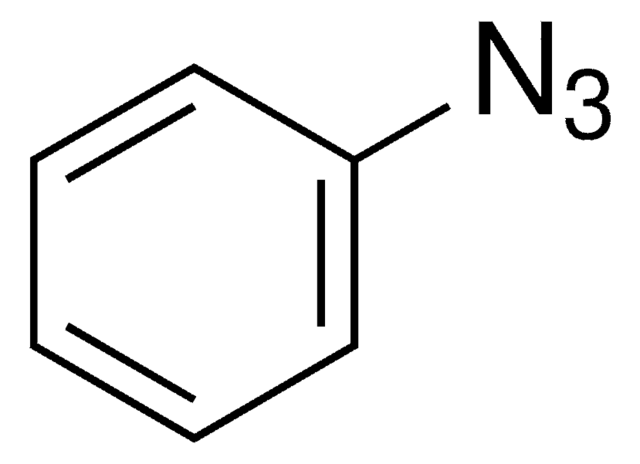
Azidobenzene solution
~0.5 M in tert-butyl methyl ether
Synonym(s):
Phenyl azide solution
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H5N3
CAS Number:
Molecular Weight:
119.12
Beilstein/REAXYS Number:
742248
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
≥95.0% (HPLC)
Quality Level
form
liquid
concentration
~0.5 M in tert-butyl methyl ether
impurities
≤2.0% water
functional group
azide
storage temp.
−20°C
SMILES string
[N-]=[N+]=Nc1ccccc1
InChI
1S/C6H5N3/c7-9-8-6-4-2-1-3-5-6/h1-5H
InChI key
CTRLRINCMYICJO-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
signalword
Danger
hcodes
Hazard Classifications
Flam. Liq. 2 - Skin Irrit. 2 - STOT RE 2
Storage Class
3 - Flammable liquids
wgk_germany
WGK 3
flash_point_f
39.2 °F
flash_point_c
4 °C
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
D Wild
Chemico-biological interactions, 82(1), 123-132 (1992-03-01)
Photolysis of arylazides produces short-lived reactive species, very likely arylnitrenium ions which bind to nucleotides and DNA and produce mutations in Salmonella. The present report shows that arylazides can be photo-activated in mammalian (V79 Chinese hamster) cells and that sister
Manabu Mizutani et al.
Biomacromolecules, 3(4), 668-675 (2002-07-09)
Photoreactive phenylazide-end-capped liquid copolymers were prepared by ring-opening copolymerization of epsilon-caprolactone (CL) and trimethylene carbonate (TMC) at an equimolar monomer feed ratio in the presence of a polyol, namely, a low-molecular-weight alcohol (di-, tri-, and tetraol) or poly(ethylene glycol) (PEG)
David Evrard et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 14(30), 9286-9291 (2008-09-10)
The electrochemical reduction of phenylazide or phenylacetylene diazonium salts leads to the grafting of azido or ethynyl groups onto the surface of carbon electrodes. In the presence of copper(I) catalyst, these azide- or alkyne-modified surfaces react efficiently and rapidly with
Mary S Rizk et al.
Biochemistry, 45(2), 543-551 (2006-01-13)
The reactive 1,2-didehydroazepine (cyclic ketenimine) intermediates produced upon photolysis of phenyl azide, 3-hydroxyphenyl azide, 3-methoxyphenyl azide, and 3-nitrophenyl azide in water and in HEPES buffer were studied by laser flash photolysis techniques with UV-vis detection of the transient intermediates. The
Jin Wang et al.
Organic letters, 9(20), 3973-3976 (2007-08-28)
Ultrafast photolysis (lambda(ex) = 308 nm) of phenyl azide in 100% formic acid produces a broadly absorbing transient within the instrument time resolution (300 fs), which is assigned to an excited state of the azide. The azide excited state fragments
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service