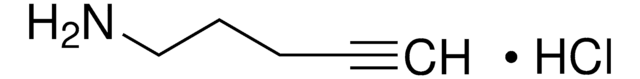715190
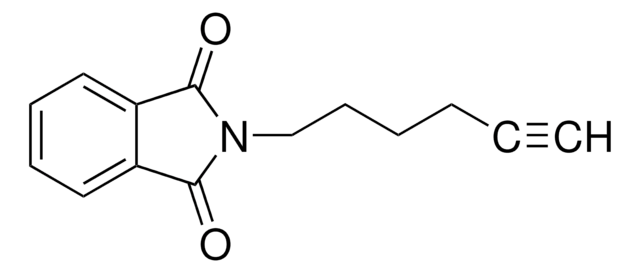
1-Amino-3-butyne
95%
Synonym(s):
3-Butyn-1-amine, 3-Butynylamine, 4-Amino-1-butyne
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C4H7N
CAS Number:
Molecular Weight:
69.11
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
95%
form
liquid
refractive index
n20/D 1.448
bp
100-103 °C
density
0.844 g/mL at 25 °C
storage temp.
2-8°C
SMILES string
NCCC#C
InChI
1S/C4H7N/c1-2-3-4-5/h1H,3-5H2
InChI key
XSBPYGDBXQXSCU-UHFFFAOYSA-N
Application
1-Amino-3-butyne, a bifunctional linker can be used:
- In Buchwald–Hartwig amination reaction of 1,2,3,4-tetrahydroacridine trifluoromethanesulfonate derivative.
- As a reagent to synthesize dialkynylamides from diacids.
- For the post-polymerization modification of poly(2-alkyl/aryl-2-oxazoline)s (PAOx) polymer by amidation of its methyl ester side chains.
- As a crosslinker which can bind with resin as well as nanocrystalline cellulose to facilitate the terminal alkyne group for further functionalizations.
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1B
Storage Class
3 - Flammable liquids
wgk_germany
WGK 3
flash_point_f
50.0 °F - closed cup
flash_point_c
10 °C - closed cup
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Buchwald-Hartwig Amination Approach for the Synthesis of Functionalized 1, 2, 3, 4-Tetrahydroacridine Derivatives.
de Sousa J, et al.
European Journal of Organic Chemistry, 2014(16), 3468-3474 (2014)
Predictive recognition of native proteins by cucurbit [7] uril in a complex mixture.
Li W, et al.
Chemical Communications (Cambridge, England), 52(55), 8537-8540 (2016)
Functional poly (2-oxazoline) s by direct amidation of methyl ester side chains.
Mees MA and Hoogenboom R
Macromolecules, 48(11), 3531-3538 (2015)
A bottom-up route to a chemically end-to-end assembly of nanocellulose fibers.
Yang H and van de Ven TGM
Biomacromolecules, 17(6), 2240-2247 (2016)
Conformationally rigid cyclic tungsten bis-alkyne complexes derived from 1, 1?-dialkynylferrocenes.
Curran TP, et al.
Journal of Organometallic Chemistry, 846(16), 24-32 (2017)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service