69723
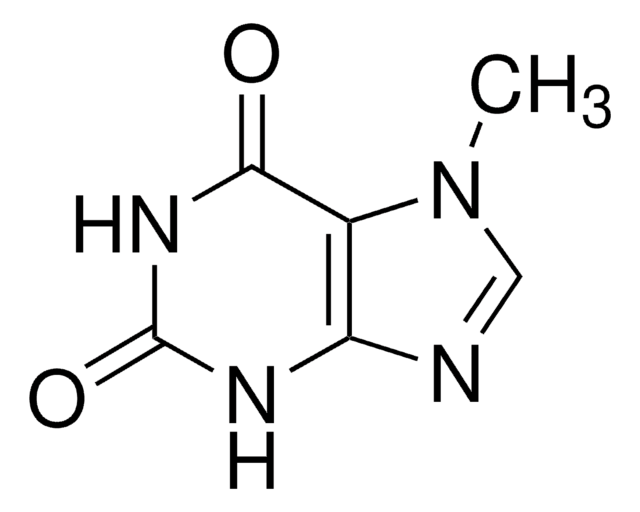
7-Methylxanthine
≥98.0% (HPLC)
Synonym(s):
2,6-Dihydroxy-7-methylpurine, Heteroxanthine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H6N4O2
CAS Number:
Molecular Weight:
166.14
Beilstein/REAXYS Number:
171027
EC Number:
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
≥98.0% (HPLC)
form
powder
mp
≥300 °C
SMILES string
Cn1cnc2NC(=O)NC(=O)c12
InChI
1S/C6H6N4O2/c1-10-2-7-4-3(10)5(11)9-6(12)8-4/h2H,1H3,(H2,8,9,11,12)
InChI key
PFWLFWPASULGAN-UHFFFAOYSA-N
Gene Information
rat ... Adora1(29290) , Adora2a(25369)
Looking for similar products? Visit Product Comparison Guide
General description
7-Methylxanthine is an oxopurine, which belongs to the class of xanthines. It may be synthesized by the reaction between 4-amino-1-methylimidazole-5-carboxamide and diethyl carbonate.
Application
7-Methylxanthine can be used as a building block to synthesize tricyclic imidazo[2,1-i]purinone derivatives as potential adenosine receptor antagonists.
Packaging
Bottomless glass bottle. Contents are inside inserted fused cone.
related product
Product No.
Description
Pricing
Storage Class
13 - Non Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Swati Sucharita Dash et al.
Current microbiology, 55(1), 56-60 (2007-06-08)
In this study, the kinetics of degradation of caffeine and related methylxanthines by induced cells of Pseudomonas sp. was performed. The kinetics data showed that degradation of caffeine, theobromine, and 7-methylxanthine followed Michealis-Menten kinetics. The values of K (m) are
K Trier et al.
The British journal of ophthalmology, 83(12), 1370-1375 (1999-11-27)
To examine a possible effect of 7-methylxanthine, theobromine, acetazolamide, or L-ornithine on the ultrastructure and biochemical composition of rabbit sclera. Groups of pigmented rabbits, six in each group, were dosed during 10 weeks with one of the substances under investigation
W A Tramontano et al.
Phytochemistry, 29(1), 31-33 (1990-01-01)
The use of a DNA alkylating agent, which induces poly(ADP-ribose) formation, has been employed to study the incorporation of [adenine 14C]NAD into pea root meristem nuclei, which is a prerequisite for poly(ADP-ribose) synthesis. The incorporation of [adenine 14C]NAD is significantly
Purines
Shaw G
Comprehensive Heterocyclic Chemistry III, 5, 499-605 (1984)
Imidazo [2, 1-i] purin-5-ones and related tricyclic water-soluble purine derivatives: potent A2A-and A3-adenosine receptor antagonists
Muller CE, et al.
Journal of Medicinal Chemistry, 45(16), 3440-3450 (2002)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service