640522
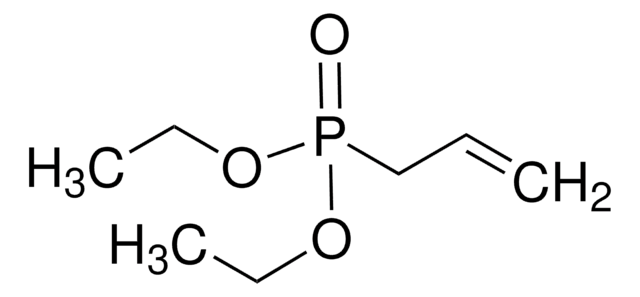
Diethyl 3-butenylphosphonate
95%
Synonym(s):
P-3-Buten-1-yl-phosphonic acid diethyl ester
About This Item
Recommended Products
assay
95%
reaction suitability
reaction type: C-C Bond Formation
bp
55 °C/0.55 mmHg (lit.)
density
1.005 g/mL at 25 °C (lit.)
functional group
allyl
phosphonate
SMILES string
CCOP(=O)(CCC=C)OCC
InChI
1S/C8H17O3P/c1-4-7-8-12(9,10-5-2)11-6-3/h4H,1,5-8H2,2-3H3
InChI key
NUDGAPYPOAQVLP-UHFFFAOYSA-N
Application
- Bicyclo[3.3.1]alkenone framework compounds by gold-catalyzed Diels-Alder reactions
- Antiviral C-5-substituted pyrimidine acyclic nucleoside phosphonates selected as human thymidylate kinase substrates
- Antimalarials via halogenation of di-Et butenylphosphonate
- Bisphosphonate enynes
Reactant for:
- Remote supramolecular control of catalyst selectivity in hydroformlyation of alkenes
- Intermolecular metal-catalyzed carbenoid cyclopropanations
- Chemoenzymatic synthesis of phosphic derivatives of carbohydrates
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 3 Oral
Storage Class
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
wgk_germany
WGK 3
flash_point_f
222.1 °F - closed cup
flash_point_c
105.6 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service