560235
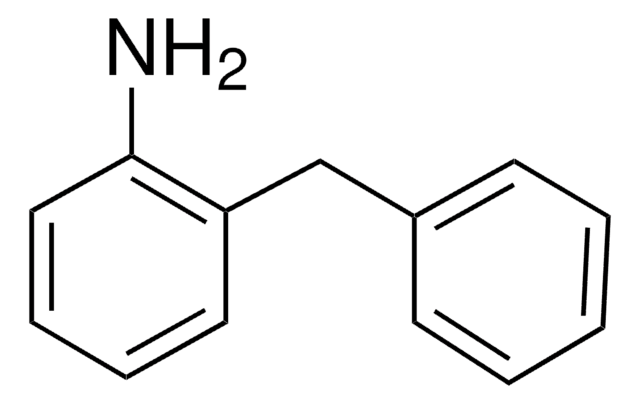
4-Benzylaniline
97%
Synonym(s):
4-Aminodiphenylmethane
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6H5CH2C6H4NH2
CAS Number:
Molecular Weight:
183.25
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
97%
form
solid
mp
35-38 °C (lit.)
SMILES string
Nc1ccc(Cc2ccccc2)cc1
InChI
1S/C13H13N/c14-13-8-6-12(7-9-13)10-11-4-2-1-3-5-11/h1-9H,10,14H2
InChI key
WDTRNCFZFQIWLM-UHFFFAOYSA-N
General description
4-Benzylaniline (4-BA) undergoes hydrochlorination in the presence of HCl to yield 4-benzylaniline hydrochloride. Crystals of 4-BA exhibit monoclinic crystal system and space group P21/c.
Application
4-Benzylaniline (4-Aminodiphenylmethane) can be used to synthesize:
- 2-amino-6-benzylbenzothiazole (SKA-7)
- ethyl 2-((4-benzylphenylamino)methylen)-malonate
- bis(4-benzylphenyl)-urea
- 3:5-dibromo-4-aminodiphenylmethane via treatment with bromine solution
- 3:5-di-iodo-4-aminodiphenylmethane via iodination reaction by using a suitable iodinating reagent[sodium iodate + potassium iodide]
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
230.0 °F - closed cup
flash_point_c
110 °C - closed cup
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
A structural and spectroscopic investigation of the hydrochlorination of 4-benzylaniline: the interaction of anhydrous hydrogen chloride with chlorobenzene.
Gibson EK, et al.
Physical Chemistry Chemical Physics, 11(2), 288-297 (2009)
"252.Some substitution reactions of 4-aminodiphenylmethane"
Waters.AW
Journal of the Chemical Society, 1060-1064 (1933)
Investigating the role of metal chelation in HIV-1 integrase strand transfer inhibitors.
Bacchi A, et al.
Journal of Medicinal Chemistry, 54(24), 8407-8420 (2011)
Naphtho [1, 2-d] thiazol-2-ylamine (SKA-31), a new activator of KCa2 and KCa3. 1 potassium channels, potentiates the endothelium-derived hyperpolarizing factor response and lowers blood pressure.
Sankaranarayanan A, et al.
Molecular Pharmacology, 75(2), 281-295 (2009)
Origin of impurities formed in a polyurethane production chain. Part 2: A route to the formation of colored impurities.
Callison J, et al.
Industrial & Engineering Chemistry Research, 51(34), 11021-11030 (2012)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service