All Photos(1)
About This Item
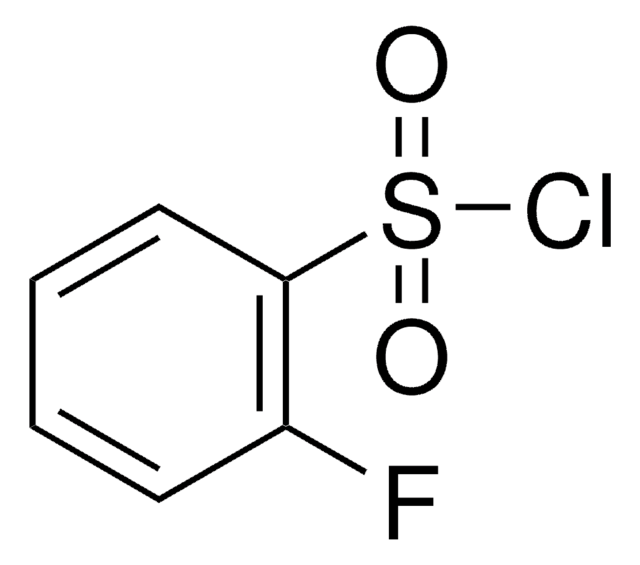
Linear Formula:
BrC6H3(F)SO2Cl
CAS Number:
Molecular Weight:
273.51
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
Recommended Products
assay
97%
mp
63-66 °C (lit.)
SMILES string
Fc1cc(Br)ccc1S(Cl)(=O)=O
InChI
1S/C6H3BrClFO2S/c7-4-1-2-6(5(9)3-4)12(8,10)11/h1-3H
InChI key
XNYBZLRIUHNRQY-UHFFFAOYSA-N
General description
4-Bromo-2-fluorobenzenesulfonyl chloride participates in the generation of tricyclic benzothiadiazepine-1,1-dioxde via pairing reactions with (S)-prolinol.
Application
4-Bromo-2-fluorobenzenesulfonyl chloride may be used to synthesize (E)-4-bromo-2-fluoro-1-styrylbenzene.
signalword
Danger
hcodes
Hazard Classifications
Skin Corr. 1B
Storage Class
8A - Combustible corrosive hazardous materials
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Palladium-Catalysed Desulfitative Heck Reaction Tolerant to Aryl Carbon?Halogen Bonds for Access to (Poly) halo-Substituted Stilbene or Cinnamate Derivatives.
Skhiri A, et al.
Synthesis (2016)
Reaction Pairing: A Diversity-Oriented Synthesis Strategy for the Synthesis of Diverse Benzofused Sultams.
Samarakoon TB, et al.
Organic Letters, 13(19), 5148-5148 (2011)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service