All Photos(1)
About This Item
Linear Formula:
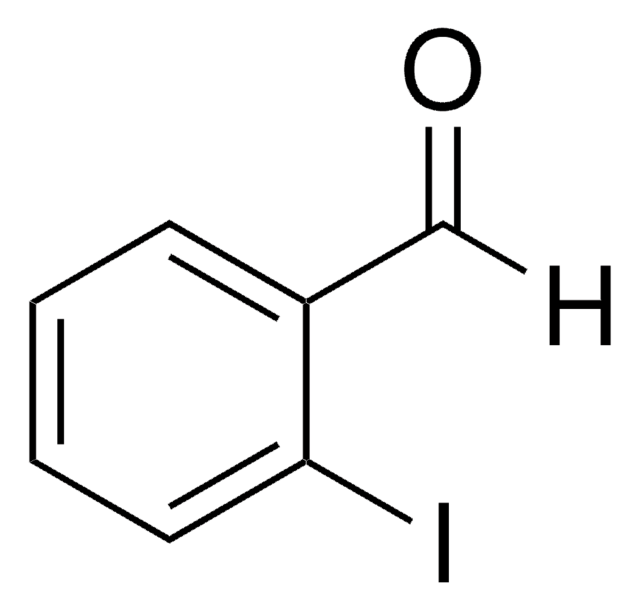
IC6H4COCH3
CAS Number:
Molecular Weight:
246.05
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
97%
refractive index
n20/D 1.618 (lit.)
density
1.72 g/mL at 25 °C (lit.)
SMILES string
CC(=O)c1ccccc1I
InChI
1S/C8H7IO/c1-6(10)7-4-2-3-5-8(7)9/h2-5H,1H3
InChI key
XDXCBCXNCQGZPG-UHFFFAOYSA-N
General description
2′-Iodoacetophenone is a halogenated aromatic ketone.
Application
2′-Iodoacetophenone (2-Iodoacetophenone) may be used in the synthesis of:
- indene derivatives
- di-(o-acetylphenyl)acetylene
- indenol derivative
Storage Class
12 - Non Combustible Liquids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Cobalt-Catalyzed Carbocyclization of o-Iodobenzaldehydes and o-Iodophenylketones with Alkynes.
Chang KJ, et al.
Organic Letters, 5(21), 3963-3966 (2003)
Kuo-Jui Chang et al.
The Journal of organic chemistry, 69(14), 4781-4787 (2004-07-03)
An efficient cobalt-catalyzed carbocylization for the synthesis of indenols and indenes and a new method for reductive decyanation are described. 2-Iodophenyl ketones and aldehydes 1a-g undergo carbocyclization with various disubstituted alkynes 2a-k in the presence of Co(dppe)I(2) and zinc powder
Charles P Casey et al.
Beilstein journal of organic chemistry, 1(1), 18-18 (2006-03-18)
The reaction of di-(o-acetylphenyl)acetylene (1) with excess dimethyl acetylenedicarboxylate (DMAD) produced bis-DMAD adducts meso-3 and rac-3. This transformation is suggested to involve thermal rearrangement of 1 to the intermediate 3,3'-dimethyl-1,1'-biisobenzofuran (A), and subsequent Diels-Alder cycloadditions of two equivalents of DMAD
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service