535486
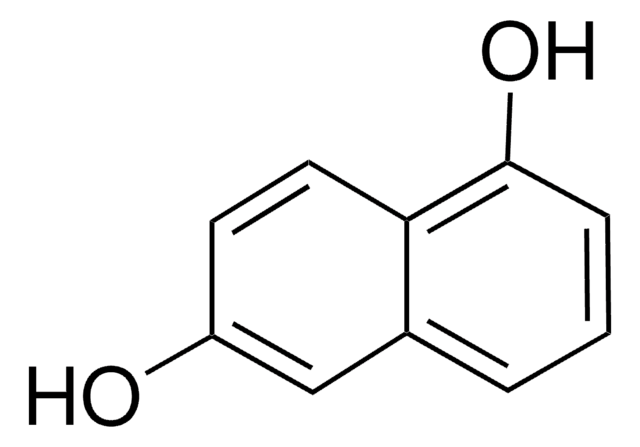
1,7-Dihydroxynaphthalene
97%
Synonym(s):
1,7-Naphthalenediol
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
C10H6(OH)2
CAS Number:
Molecular Weight:
160.17
Beilstein/REAXYS Number:
1908499
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
97%
mp
180-184 °C (lit.)
SMILES string
Oc1ccc2cccc(O)c2c1
InChI
1S/C10H8O2/c11-8-5-4-7-2-1-3-10(12)9(7)6-8/h1-6,11-12H
InChI key
ZUVBIBLYOCVYJU-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 2
flash_point_f
485.6 °F - closed cup
flash_point_c
252 °C - closed cup
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Anna Klimek-Turek et al.
Molecules (Basel, Switzerland), 25(21) (2020-11-04)
In this manuscript, the retention of aromatic hydrocarbons with polar groups has been compared for systems with various nonpolar columns of the types from C3 to C18 and different mobile phases composed of methanol, acetonitrile, or tetrahydrofuran as modifiers. The
Mårten Jacobsson et al.
Journal of medicinal chemistry, 49(6), 1932-1938 (2006-03-17)
The antiproliferative activity of the 14 isomeric monoxylosylated dihydroxynaphthalenes has been tested in vitro toward normal HFL-1 and 3T3 A31 cells as well as transformed T24 and 3T3 SV40 cells. The antiproliferative effect toward HFL-1 cells was correlated with the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service