All Photos(1)
About This Item
Empirical Formula (Hill Notation):
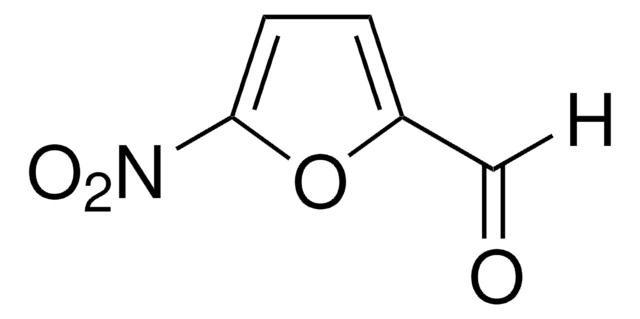
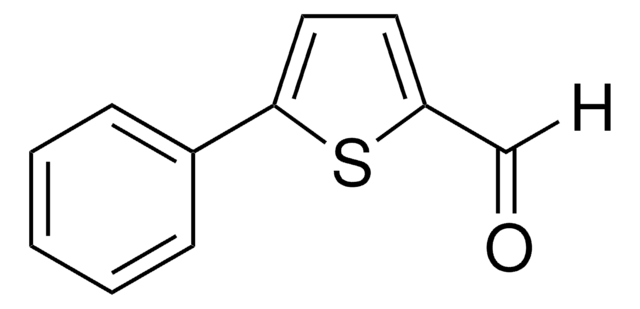
C11H8O2
CAS Number:
Molecular Weight:
172.18
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
96%
mp
29-33 °C (lit.)
functional group
aldehyde
phenyl
SMILES string
O=Cc1ccc(o1)-c2ccccc2
InChI
1S/C11H8O2/c12-8-10-6-7-11(13-10)9-4-2-1-3-5-9/h1-8H
InChI key
BMJHNNPEPBZULA-UHFFFAOYSA-N
General description
5-Phenyl-2-furaldehyde can be prepared from phenylfuran, via formylation. It undergoes electrochemical reduction on a dropping Hg electrode to afford anion radicals in DMF.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
230.0 °F - closed cup
flash_point_c
110 °C - closed cup
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Furan derivatives. XXXI.< x,/MJnsaturated ketones of the phenylfuran series.
Frimm R, et al.
Chem. Zeit., 27(1), 101-106 (1973)
Cathodic reduction of substituted 5-phenyl-2-furaldehydes in dimethylformamide.
Cernak J, et al.
Chemical Papers, 34(6), 788-792 (1980)
Renzo P Zanocco et al.
PloS one, 13(7), e0200006-e0200006 (2018-07-03)
In this study, we report the synthesis and the photochemical behavior of a series of new "click-on" fluorescent probes designed to detect singlet oxygen. They include a highly fluorescent chemical structure, an aryloxazole ring, linked to a furan moiety operating
Kyungsil Yoon et al.
Cells, 8(3) (2019-03-15)
Chicken ovalbumin upstream promoter-transcription factor I (COUP-TFI) is an orphan receptor and member of the nuclear receptor superfamily. Among a series of methylene substituted diindolylmethanes (C-DIMs) containing substituted phenyl and heteroaromatic groups, we identified 1,1-bis(3'-indolyl)-1-(4-pyridyl)-methane (DIM-C-Pyr-4) as an activator of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service