All Photos(1)
About This Item
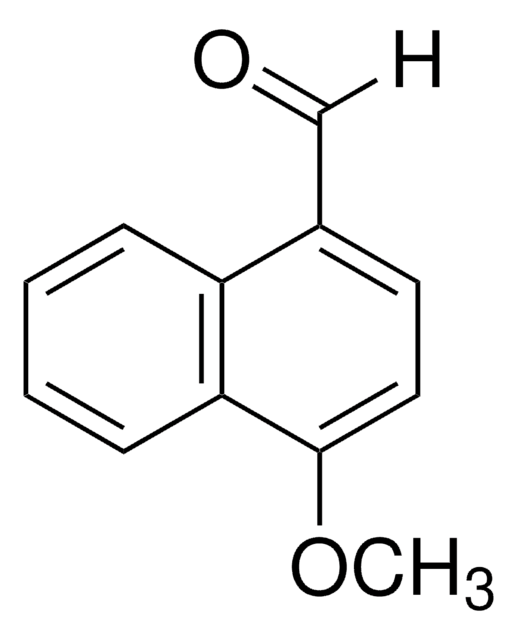
Linear Formula:
CH3C10H6CHO
CAS Number:
Molecular Weight:
170.21
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
Recommended Products
assay
97%
mp
32-36 °C (lit.)
SMILES string
Cc1ccc(C=O)c2ccccc12
InChI
1S/C12H10O/c1-9-6-7-10(8-13)12-5-3-2-4-11(9)12/h2-8H,1H3
InChI key
LANRGTXVAPBSIA-UHFFFAOYSA-N
General description
4-Methyl-1-naphthaldehyde is a monoaldehyde. It is obtained along with 1-methyl-2-naphthaldehyde from 1-methylnaphthalene, via formylation.
Application
4-Methyl-1-naphthaldehyde may be used in the synthesis of methyl 2-phenyl-3-( 4-methyl-1-naphthyl)propenoate and (Z/E)-2-amino-4,6-dimethyl-5-[(4-methyl-1-naphthyl)methylene]-5H-cyclopenta[b]pyridine-3,7-dicarbonitrile.
Storage Class
13 - Non Combustible Solids
wgk_germany
WGK 3
flash_point_f
230.0 °F - closed cup
flash_point_c
110 °C - closed cup
ppe
Eyeshields, Gloves, type N95 (US)
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
The influence of aromatic compound protonation on the regioselectivity of Gattermann-Koch formylation.
Tanaka M, et al.
Chemical Communications (Cambridge, England), 2, 159-160 (1996)
S Amin et al.
Chemical research in toxicology, 1(6), 349-355 (1988-11-01)
Previous studies have shown that 5-methylchrysene (5-MeC) is more carcinogenic on mouse skin than the other methylchrysenes and that the structural requirements favoring tumorigenicity of methylated polynuclear aromatic hydrocarbons are the presence of a bay region methyl group and free
Stéphanie Kolb et al.
European journal of medicinal chemistry, 45(3), 896-901 (2009-12-09)
We report herein the synthesis of 5-substituted [1]pyrindine derivatives and the evaluation of their antiproliferative properties on HeLa cells, a cervical carcinoma tumor cell line, and on the melanoma A2058 cell line. The most efficient compounds display cytotoxicity against tumor
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service