523968
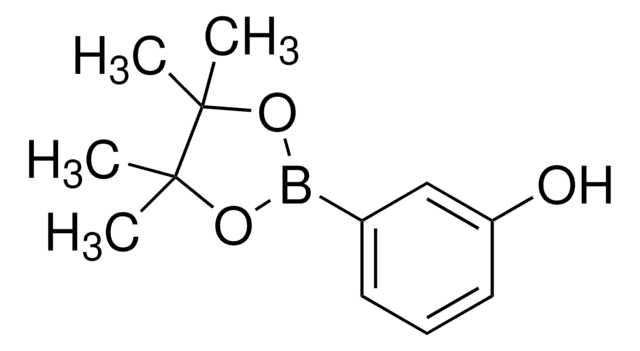
3-Hydroxyphenylboronic acid
≥95.0%
Synonym(s):
3-Hydroxybenzeneboronic acid, m-Hydroxybenzeneboronic acid
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
HOC6H4B(OH)2
CAS Number:
Molecular Weight:
137.93
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
≥95.0%
mp
210-213 °C (dec.) (lit.)
SMILES string
OB(O)c1cccc(O)c1
InChI
1S/C6H7BO3/c8-6-3-1-2-5(4-6)7(9)10/h1-4,8-10H
InChI key
WFWQWTPAPNEOFE-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
3-Hydroxyphenylboronic acid (3-HPBA) can be used as a reagent:
- In Suzuki-Miyaura coupling reactions with aryl halides for the formation of C-C bond in the presence of Pd catalyst.
- To synthesize boron/nitrogen-doped polymer nano/microspheres by hydrothermal polymerization with formaldehyde and ammonia.
- To prepare carbon quantum dots based on 3-HPBA as selective fructose sensor.
- In the development of modified electrodes for electrochemical biosensors.
Footnote
Contains varying amounts of anhydride
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis of hyperbranched polythiophene with a controlled degree of branching via catalyst-transfer Suzuki-Miyaura coupling reaction
Segawa Y, et al.
Polym. Chem., 4(4), 1208-1215 (2013)
Diana M A Crista et al.
Journal of fluorescence, 29(1), 265-270 (2019-01-07)
The selective fluorescence sensing of fructose was achieved by fluorescence quenching of the emission of hydrothermal-synthesized carbon quantum dots prepared by 3-hydroxyphenylboronic acid. Quantification of fructose was possible in aqueous solutions with pH of 9 (Limit of Detection LOD and
Facile synthesis of monodisperse bulk boron-and nitrogen-doped carbon nano/microspheres
Zhao J, et al.
Journal of Material Chemistry A, 6(46), 23780-23786 (2018)
Pierangelo Bellio et al.
Life sciences, 241, 117116-117116 (2019-12-04)
LexA protein is a transcriptional repressor which regulates the expression of more than 60 genes belonging to the SOS global regulatory network activated by damages to bacterial DNA. Considering its role in bacteria, LexA represents a key target to counteract
Mingyan Zhu et al.
ACS combinatorial science, 14(2), 124-134 (2011-12-21)
As a continuation of our previous report (J. Comb. Chem.2010, 12, 548-558), we accomplished the diversity-oriented synthesis of polyheterocyclic small-molecule library with privileged benzopyran substructure. To ensure the synthetic efficiency, we utilized the solid-phase parallel platform and the fluorous-tag-based solution-phase
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service