49362
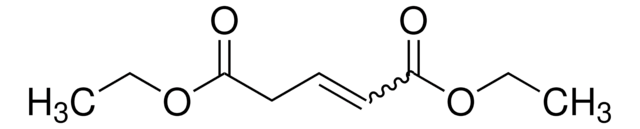
Dimethyl glutaconate
≥95.0% (GC)
Synonym(s):
Dimethyl 2-pentenoate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3OOCCH2CH=CHCOOCH3
CAS Number:
Molecular Weight:
158.15
Beilstein/REAXYS Number:
1724169
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
≥95.0% (GC)
refractive index
n20/D 1.452
density
1.124 g/mL at 20 °C (lit.)
functional group
ester
SMILES string
COC(=O)C\C=C\C(=O)OC
InChI
1S/C7H10O4/c1-10-6(8)4-3-5-7(9)11-2/h3-4H,5H2,1-2H3/b4-3+
InChI key
SKCGFFOFYXLNCG-ONEGZZNKSA-N
General description
Dimethyl glutaconate, also known as dimethyl 2-pentenoate, is a diester. It reacts with salicaldehyde in the presence of piperidine to form a coumarin-fused electron deficient diene.
Application
Dimethyl glutaconate may be used in the synthesis of:
- dimethyl 3-[(Z)-1-propenyl]glutarate
- phenanthridinone derivatives
- substituted benzenes
Components
composition: ~83% trans + ~17% cis
hcodes
pcodes
Hazard Classifications
Aquatic Chronic 3
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Electron Deficient Dienes. 2. One Step Synthesis of a Coumarin-Fused Electron Deficient Diene and its Inverse Electron Demand Diels-Alder Reactions with Enamines.
Bodwell GJ, et al.
Synlett, 4, 477-479 (1999)
One-Pot Synthesis of Phenanthridinones by Using a Base-Catalyzed/Promoted Bicyclization of a, ?-Unsaturated Carbonyl Compounds with Dimethyl Glutaconate.
Li L, et al.
European Journal of Organic Chemistry, 2015(22), 4892- 4899 (2015)
A convenient method for the preparation of 3-substituted glutarate diesters.
Leotta III, et al.
The Journal of Organic Chemistry, 59(7), 1946-1946 (1994)
Base-Catalyzed Efficient Tandem [3+3] and [3+2+1] Annulation-Aerobic Oxidative Benzannulations.
Diallo A, et al.
Organic Letters, 14(22), 5776-5779 (2012)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service