467030
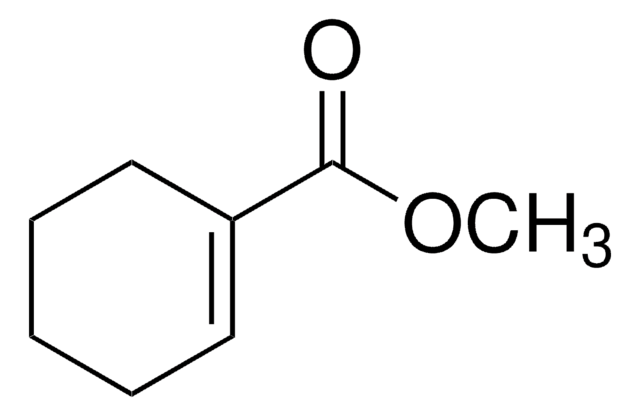
1-Cyclohexene-1-carboxaldehyde
97%
Synonym(s):
Δ1-Tetrahydrobenzaldehyde, 1-Cyclohexenecarboxaldehyde, 1-Cyclohexenylaldehyde, 1-Formyl-1-cyclohexene, 1-Formylcyclohexene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6H9CHO
CAS Number:
Molecular Weight:
110.15
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
97%
bp
61 °C/10 mmHg (lit.)
density
0.966 g/mL at 25 °C (lit.)
functional group
aldehyde
SMILES string
O=CC1=CCCCC1
InChI
1S/C7H10O/c8-6-7-4-2-1-3-5-7/h4,6H,1-3,5H2
Inchi Key
OANSOJSBHVENEI-UHFFFAOYSA-N
General description
1-Cyclohexene-1-carboxaldehyde is an α,β-unsaturated aldehyde. It participates in the synthesis of benzopyrans.
Application
1-Cyclohexene-1-carboxaldehyde may be used in the synthesis of azomethine imines.
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
149.0 °F - closed cup
flash_point_c
65 °C - closed cup
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Feng Shi et al.
Tetrahedron letters, 50(28), 4067-4070 (2010-02-18)
A [3+2] 1,3-dipolar cycloaddition reaction of arynes with stable azomethine imines has been developed. The reaction rapidly assembles tricyclic pyrazoloindazolone derivatives in moderate yields under mild reaction conditions.
Environmentally benign, one-pot synthesis of pyrans by domino Knoevenagel/6p-electrocyclization in water and application to natural products.
Jung EJ, et al.
Green Chemistry, 12(11), 2003-2011 (2010)
Efficient and general method for the synthesis of benzopyrans by ethylenediamine diacetate-catalyzed reactions of resorcinols with α, β-unsaturated aldehydes. One step synthesis of biologically active (?)-confluentin and (?)-daurichromenic acid.
Lee YR, et al.
Tetrahedron Letters, 46(44), 7539-7543 (2005)
Yi Wang et al.
Plant, cell & environment, 44(2), 559-573 (2020-11-21)
In plants, cellular lipid peroxidation is enhanced under low nitrogen (LN) stress; this increases the lipid-derived reactive carbonyl species (RCS) levels. The cellular toxicity of RCS can be reduced by various RCS-scavenging enzymes. However, the roles of these enzymes in
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service