All Photos(1)
About This Item
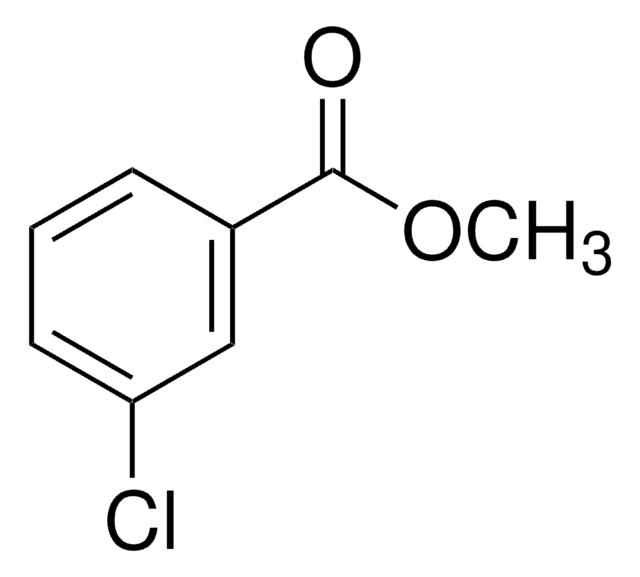
Linear Formula:
ClC6H4CO2CH3
CAS Number:
Molecular Weight:
170.59
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
≥98%
form
liquid
refractive index
n20/D 1.536 (lit.)
bp
86-88 °C/2.5 mmHg (lit.)
density
1.191 g/mL at 25 °C (lit.)
SMILES string
COC(=O)c1ccccc1Cl
InChI
1S/C8H7ClO2/c1-11-8(10)6-4-2-3-5-7(6)9/h2-5H,1H3
InChI key
JAVRNIFMYIJXIE-UHFFFAOYSA-N
General description
Methyl 2-chlorobenzoate, a methyl 2-halobenzoate, is an ester. It can be synthesized from 2-chlorobenzoyl chloride. Its reduction with NaBH4 in diglyme at 162°C affords 2-chlorobenzyl alcohol.
Application
Methyl 2-chlorobenzoate may be used in the synthesis of various quinazolinone derivatives. It was used as starting reagent in the synthesis of 2-chlorobenzohydrazide.
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
228.2 °F - closed cup
flash_point_c
109.00 °C - closed cup
ppe
Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Navin B Patel et al.
Scientia pharmaceutica, 78(2), 171-193 (2010-12-24)
In attempt to find new pharmacologically active molecules, we report here the synthesis and in vitro antimicrobial activity of various 3-(1,3,4-oxadiazol-2-yl)-quinazolin-4(3H)-ones. The antimicrobial activity of title compounds were examined against two gram positive bacteria (S. aureus, S. pyogenes), two gram
Reductions of Carboxylic Acids and Esters with NaBH4 in Diglyme at 162?C.
Zhu H-J and Pittman Jr CU.
Synthetic Communications, 33(10), 1733-1750 (2003)
Nicholas R Larson et al.
Journal of medical entomology, 57(1), 187-191 (2019-09-10)
Common bed bug Cimex lectularius (L.) (Hemiptera: Cimicidae) infestations are on the rise and due to the development of pesticide resistance they are becoming more difficult to control, affordably. We evaluated a naturally occurring compound methyl benzoate (MB) and related
Cheng Huang et al.
Chemical communications (Cambridge, England), (47)(47), 6333-6335 (2008-12-03)
We have developed a general and highly efficient copper-catalyzed method for synthesis of quinazoline and quinazolinone derivatives, the target products were obtained in good to excellent yields via cascade reactions of amidine hydrochlorides with substituted 2-halobenzaldehydes, 2-halophenylketones, or methyl 2-halobenzoates
Yan Feng et al.
Scientific reports, 8(1), 7902-7902 (2018-05-23)
Benzyl methyl ester, also known as methyl benzoate (MB), is a volatile organic compound that exists naturally as a floral fragrance in many plants. Our behavioral bioassays show that MB and some of its naturally occurring and synthetic analogs kill
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service