All Photos(1)
About This Item
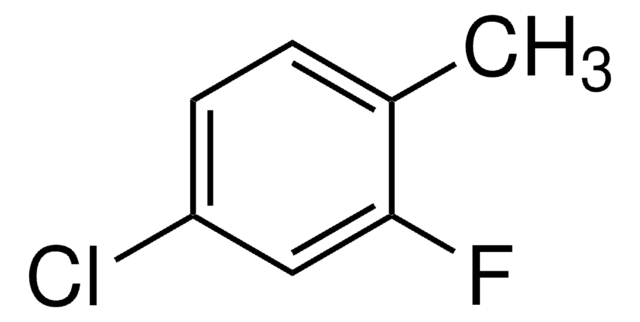
Linear Formula:
CH3C6H3(Cl)F
CAS Number:
Molecular Weight:
144.57
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
Recommended Products
assay
98%
refractive index
n20/D 1.4995 (lit.)
bp
157-159 °C/752 mmHg (lit.)
density
1.188 g/mL at 25 °C (lit.)
SMILES string
Cc1cc(Cl)ccc1F
InChI
1S/C7H6ClF/c1-5-4-6(8)2-3-7(5)9/h2-4H,1H3
InChI key
JOXXHDGUTVUBDL-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
3 - Flammable liquids
wgk_germany
WGK 3
flash_point_f
120.2 °F - closed cup
flash_point_c
49 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
K L Shepard et al.
Journal of medicinal chemistry, 34(10), 3098-3105 (1991-10-01)
For several decades a tantalizing goal for the treatment of primary open-angle glaucoma has been the development of a topically active carbonic anhydrase inhibitor. Recent results from several research groups indicate that considerable progress has been made toward this objective.
Dorus Heijnen et al.
Organic letters, 17(9), 2262-2265 (2015-04-15)
The palladium-catalyzed direct cross-coupling of a range of organic chlorides and bromides with the bifunctional C(sp(3))-(trimethylsilyl)methyllithium reagent is reported. The use of Pd-PEPPSI-IPent as the catalyst allows for the preparation of structurally diverse and synthetically versatile benzyl- and allylsilanes in
H Kanno et al.
Chemical & pharmaceutical bulletin, 40(8), 2049-2054 (1992-08-01)
New 1,4-dihydropyridine derivatives bearing a 4-(disubstituted phenyl) ring and an aminoethyl ester or an amino-2,2-dimethyl-propyl ester were synthesized and their antihypertensive activities were examined in normotensive rats and spontaneously hypertensive rats. The effects of phenyl substituents and ester groups on
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service