All Photos(1)
About This Item
Empirical Formula (Hill Notation):
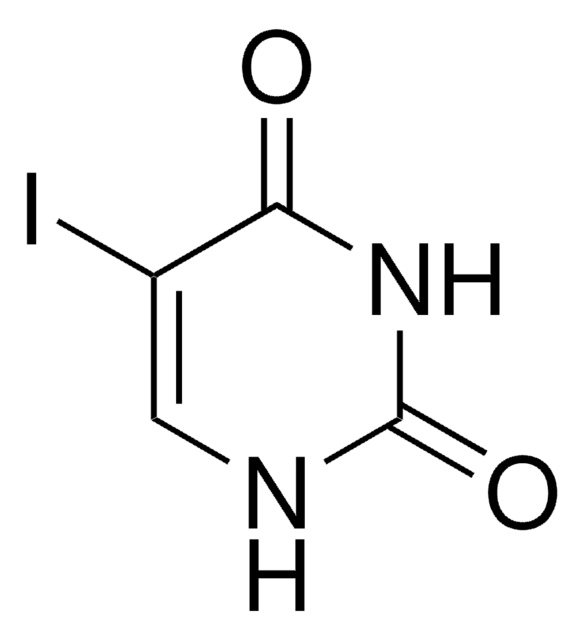
C7H10N2O3
CAS Number:
Molecular Weight:
170.17
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
99%
mp
51-54 °C (lit.)
SMILES string
COc1cc(OC)nc(OC)n1
InChI
1S/C7H10N2O3/c1-10-5-4-6(11-2)9-7(8-5)12-3/h4H,1-3H3
InChI key
RJVAFLZWVUIBOU-UHFFFAOYSA-N
General description
2,4,6-Trimethoxypyrimidine is a pyrimidine derivative. One of the methods reported for its synthesis is by the reaction of 2,4,6-trichloropyrimidine with sodium ethoxide at 70-100°C. Its transformation into 1,6-dihydro-2,4-dimethoxy-1-methyl-6-oxopyrimidine by Hilbert-Johnson reaction has been reported.
Application
2,4,6-Trimethoxypyrimidine may be used as a model compound in a study to determine the qualitative composition of mixtures formed during the methylation of barbituric acid and its derivatives by diazomethane.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Solvation effects in the methylation of barbituric acid and its derivatives by diazomethane.
Krasnov KA, et al.
Chemistry of Heterocyclic Compounds, 23(11), 1218-1221 (1987)
Chemistry of heterocyclic compounds. 29. Synthesis and reactions of multihetero macrocycles possessing 2, 4-pyrimidino subunits connected by carbon-oxygen and/or-sulfur linkages.
Newkome GR, et al.
The Journal of Organic Chemistry, 43(17), 3362-3367 (1978)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service