All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6F4N2
CAS Number:
Molecular Weight:
176.07
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
99%
form
solid
mp
66-68 °C (lit.)
functional group
fluoro
nitrile
SMILES string
Fc1nc(F)c(F)c(C#N)c1F
InChI
1S/C6F4N2/c7-3-2(1-11)4(8)6(10)12-5(3)9
InChI key
JXISJBVJNUKKBK-UHFFFAOYSA-N
General description
2,3,5,6-Tetrafluoro-4-pyridinecarbonitrile is a perfluorinated heteroaromatic compound. 2,3,5,6-Tetrafluoro-4-pyridinecarbonitrile (tetrafluoro-4-cyanopyridine) reacts with 1,3-dicarbonyl systems to yield the corresponding [5,6]-ring fused furo derivatives. 2,3,5,6-Tetrafluoro-4-pyridinecarbonitrile (4-cyanotetrafluoropyridine) reacts with amidines to yield [6,6]-fused pyrimidinopyridine system via nucleophilic substitution at the C-3 position of the pyridine ring followed by intramolecular cyclization onto the pendant cyano group. Reaction of 2,3,5,6-tetrafluoro-4-pyridinecarbonitrile (4-cyanotetrafluoropyridine) with sulfur centred nucleophiles has been reported.
Application
2,3,5,6-Tetrafluoro-4-pyridinecarbonitrile may be used in the preparation of 2-anilino-3,5,6-trifluoroisonicotinonitrile.
signalword
Warning
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Annelation of perfluorinated heteroaromatic systems by 1, 3-dicarbonyl derivatives.
Cartwright MW, et al.
Tetrahedron, 66(17), 3222-3227 (2010)
Pyrido [3, 2-b][1, 4] oxazine and pyrido [2, 3-b][1, 4] benzoxazine systems from tetrafluoropyridine derivatives.
Sandford G, et al.
Journal of Fluorine Chemistry (2014)
Reactions of 4-substituted tetrafluoropyridine derivatives with sulfur nucleophiles: S< sub> N</sub> Ar and annelation processes.
Fox MA, et al.
Journal of Fluorine Chemistry, 143, 148-154 (2012)
Imidazopyridine and pyrimidinopyridine systems from perfluorinated pyridine derivatives.
Cartwright MW, et al.
Tetrahedron, 63(30), 7027-7035 (2007)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service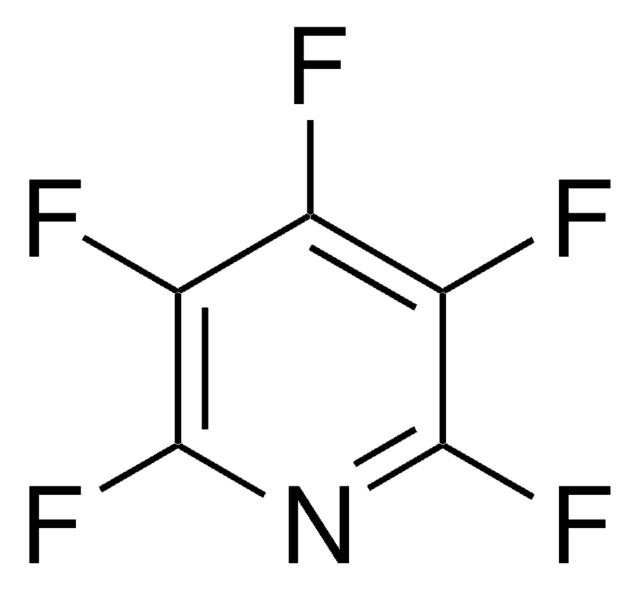




![1-Chloromethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate) >95% in F+ active](/deepweb/assets/sigmaaldrich/product/structures/206/487/53d52ee5-ef71-4e9a-9bc8-938b68b98d5d/640/53d52ee5-ef71-4e9a-9bc8-938b68b98d5d.png)